Introduction
HF is a mini-organ formed with mesenchymal-epithelial interaction (Schmidt-Ullrich & Paus, 2005). HF development and cycle are depended on signaling between its epidermal and dermal components. Especially, dermal organizing center, DP, is both chemical and physical niche for epithelial stem cells. DP plays an important role in HF induction and cycling by controlling self-renewal and differentiation of epithelial stem cell (Zhang et al., 2009). During HF development, secreted unknown Wnt ligands are considered to induce epidermal stem cells to switch to a hair induction fate (Chen et al., 2012). Indeed, canonical Wnt signaling in the DP is essential for HF formation (Alonso & Fuchs, 2003).
Molecular insights into hair development: a focus on Wnt signaling
1. Basic insights of Wnt signaling pathway
Wnt is a short-range secreted signaling molecule that regulates the development of multiple organ systems. Wnt ligands bind to their receptors, Frizzled (FZD) and low density lipoprotein receptor related protein (LRP) family, that activate a canonical signaling pathway that induces stabilization of β-catenin protein. Stabilized β-catenin is translocated into the nucleus, in which β-catenin interacts with T cell factor/lymphoid enhancer binding factor (TCF/LEF) transcription factor to target gene for activation (Bejsovec, 2000; Wodarz & Nusse, 1998). Research revealed that bone morphogenetic protein 4 (BMP4) and sonic hedgehog (SHH) are target genes of β-catenin, and have pivotal roles in hair development and regeneration (Huelsken et al., 2001) indicating that Wnt/β-catenin signaling is closely connected with hair biology.
2. Wnt signaling in hair cycle
Various Wnt ligands are associated with hair cycling. During telogen, Wnt-responding stem cells in the bulge produce Wnt1, Wnt4, and Wnt7b. During telogen to anagen transition, Wnt6, Wnt10a and Wnt10b are strongly expressed in DP. Whereas, during anagen, other Wnts including Wnt5a and Wnt5b are mainly expressed in peripheral layers of DP. Furthermore, hair stem cells produce autocrine Wnts that specify the positional identity of cells residing within the HF (Hsu et al., 2011). Therefore, Wnt regulates not only anagen initiation in DP, but also maintenance of HF stem cells during telogen.
3. Wnt signaling in HF development
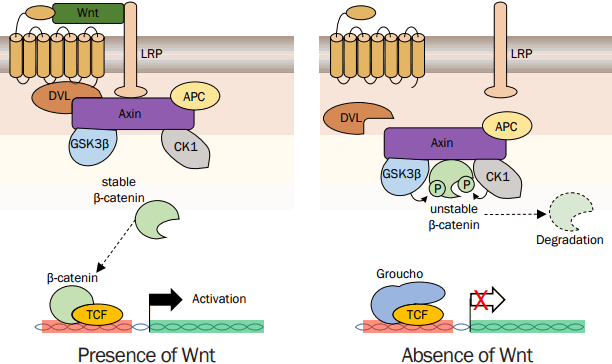
Wnt signaling is one of the fundamental mechanisms that regulate embryonic development and tissue homeostasis (MacDonald et al., 2009; Merrill, 2012). As shown in Figure 1, in the absence of Wnt, the cytoplasmic β-catenin protein is constantly degraded by the action of the Axin complex. Axin complexes include the scaffolding protein Axin, the tumor suppressor adenomatous polyposis coli (APC), casein kinase 1 (CK1), and glycogen synthase kinase 3 (GSK3). CK1 and GSK3 phosphorylate the amino terminal region of β-catenin sequentially result in conformational change. The conformational change of β-catenin helps regulate its binding to beta-transducin repeat containing E3 ubiquitin protein ligase (BTRC), subsequent β-catenin ubiquitination and proteasomal degradation. This cytoplasmic degradation of β-catenin prevents the nuclear translocation, and target genes are repressed by the histone deacetylase (HDAC)/DNA-bound TCF/LEF complex. The canonical Wnt signaling transduction initiated by Wnt ligand that binds to a seven-pass transmembrane FZD receptor and its co-receptor, LRP6 or its close relative LRP5. The formation of a likely Wnt-FZD-LRP6 complex together with the recruitment of the scaffolding protein Dishevelled (DVL) results in LRP6 phosphorylation and activation and the recruitment of the Axin complex to the receptors. These events lead to inhibition of Axin-mediated β-catenin phosphorylation and thereby to the stabilization of β-catenin, which accumulates and localizes to the nucleus to engage TCF/LEF transcription factors and activates Wnt target gene expression.
It is widely accepted that the canonical Wnt signaling is required for HF morphogenesis and differentiation (Lim & Nusse, 2013). Activation of canonical Wnt signaling and subsequence stabilization and nuclear localization of β-catenin are critical for onset of anagen phase. Wnt/β-catenin, temporally and spatially regulates the growth of stem cells and progeny cells during hair cycle. Proliferation in the matrix also depends on Wnt signaling (Lim & Nusse, 2013). Recently, using live imaging analysis of mouse model harboring the high sensitive Wnt reporter, the distinct activation is visualized in some HF stem cells in bulge region at telogen-to-anagen transition phase (Deschene et al., 2014). Furthermore, Wnt10b and Wnt16 expression are dominantly localized to new hair growth foci (Deschene et al., 2014). Interestingly, Wnt10b ligand-mediated β-catenin activation is also involved in development of types of progenitor cells including melanocyte stem cells and follicular outer root sheath (ORS) cells (Ye et al., 2013).
Recent works focus attention on the role of Wnt/β-catenin signaling in the DP for HF induction and growth. Zhou L and his colleagues were reported that Wnt/β-catenin signaling accelerates the spontaneous growth of CD133+ DP cells and matrix keratinocyte following HF differentiation (Zhou et al., 2016a). In following study, they further demonstrated that Wnt ligands produced by CD133+ DP cells play an important role in postnatal hair growth by maintaining the growth onset of DP cells and mediating the signaling cross-talk in hair bulge region (Zhou et al., 2016b). However, it is unclear how the signals act upon HF stem cells and progenitor cells in bulge region control cell type-specific development to organize growth during the hair cycle. Recent studies on Wnt signaling in DP cells are very important in this respect. It has been reported that HF quiescence is maintained dependent on BMP signaling (Plikus et al., 2008). Temporally activations of BMP2/4, Noggin, a BMP antagonist, and Wnt signals elaborately regulate the development of HF bulge stem cell activation and growth during hair cycle (Plikus et al., 2008). It could be inferred that BMP signaling is overcome by activating Wnt signals, proliferation begins first in the hair germ possibly due the proximity of these cells to the DP which is the likely source of these signals (Rompolas & Greco, 2014).
Conclusion
It has been cleared that Wnt/β-catenin signaling pathway is important for reciprocal interaction and communication between dermal/mesenchymal and epidermal components in hair bulb at the bottom of the HF. Further studies are needed to elucidate the mechanism of cross-talking between Wnt/β-catenin signal and other signaling pathways that may act upon maintenance of the HF homeostasis.