요약목적본 연구의 목적은 17가지 에센셜 오일(EO)이 사용 농도 및 추출 부위에 따른 항균 활성을 포함하여 Escherichia coli (E. coli)에 미치는 영향을 조사하는 것입니다.
방법모든 17개의 EO는 0.5% (v/v) 및 1% (v/v) 농도에서 단계적 희석에 의해 제조되었습니다. EO을 E. coli 배양액과 함께 96-well plates에 분배하고 18시간 동안 인큐베이션 했습니다. 그런 다음 마이크로플레이트 판독기를 사용하여 흡광도를 측정했습니다.
AbstractPurposeThe purpose of this study was to investigate the effects of 17 essential oils (EOs) on Escherichia coli (E. coli), including their antibacterial activity according to the concentration used and its extraction site.
MethodsAll 17 EOs were prepared by step dilution at concentrations of 0.5% (v/v) and 1% (v/v). EOs were dispensed into 96-well plates with bacterial culture aliquots and incubated for 18 h; then, their absorbance was measured using a microplate reader.
ResultsCulture absorbance showed that thyme white (TM), palmarosa (PR) and rosemary verbenone (RM) at concentrations of 0.5% (v/v) and 1% (v/v) showed high antibacterial activity, similar to that of ampicillin. Melisa true (MS), RM, PR, lavender Bulgarian (LV), lemon (LM), peppermint premium (PM) and eucalyptus bluegum (EC) also showed increased antibacterial activity in a concentration-dependent manner. Finally, TM, MS, RM, PR, and lemongrass (LG) extracted from leaves showed higher antibacterial activity than extracts from other organs.
ConclusionTM extracted from leaves showed an excellent antibacterial effect; it exhibited bacterial growth at the same level of ampicillin. It is a natural substance that suppresses the action of bacteria in the inflammatory reaction of the skin and that may be used as a raw material for customized cosmetics.
中文摘要结果 培养吸光度显示百里香(thyme white, TM)、 玫瑰草(palmarosa, PR)和马鞭草酮迷迭香(rosemary verbenone, RM)在浓度为0.5% (v/v)和1% (v/v)时,显示出与氨苄青霉素相似的高抗菌活性。Melisa true (MS)、RM、PR、保加利亚薰衣草(lavender bulgarian, LV)、柠檬(lemon, LM)、薄荷特级(peppermint premium, PM)和蓝胶尤加利(eucalyptus blue gum, EC)也以浓度依赖性方式显示出提高其抗菌活性。最后,从叶子中提取的TM、MS、RM、PR和柠檬草(lemongrass, LG)显示出比其他器官提取物具有更高的抗菌活性。
Introduction
Escherichia coli, a gram-negative bacterium, is an opportunistic pathogen in humans (Bondarenko et al., 2018) that is commonly present in the human gut. Though E. coli is generally a harmless symbiotic bacterium (Croxen et al., 2013) it may cause urinary tract infections, gastrointestinal infections, and other local tissue and organ infections under certain conditions (Lee et al., 2020b; Wood, 2009). Essential oils (EOs), used in aromatherapy, are highly concentrated oils (Dunning, 2013) extracted from the flowers, fruits, stems, roots, and resins of plants. Frequently, EOs have various antibacterial, antifungal, antiviral, anti-inflammatory, antioxidant, and insect repellent effects as well as hormonal action (Ali et al., 2015). Research on the antibacterial effects of EOs is being actively conducted in various fields (Jo et al., 2018; Park & Kang, 2020; Wang et al., 2020; Wu et al., 2019), but there are not many comparisons on the antibacterial activity of several types of EOs against E. coli.
In this study, we investigated the antibacterial activity of 17 different EOs, widely used in daily life, against E. coli. The antibacterial activity of each type of EO, at concentrations of 0.5% and 1% (v/v), was tested; in addition, the antibacterial activity of different plant organ extracts was studied. Our data suggest that EOs can be actively used as natural antibacterial materials in related industries (food and cosmetics manufacturing).
Materials and Methods1. Materials1) BacteriaThe E. coli strain used in this study (KCTC, 11643) was purchased from the Korean Collection for Type Cultures (KCTC).
2) EOsIn these experiments, 17 of La Selection products from The Certification Academy for Holistic Aromatherapy were used (Table 1).
2. Methods1) Culture conditions
E. coli strains were subcultured at 37℃ for 18 h on nutrient agar (NA; Difco™, France), and diluted in nutrient broth (NB) to match 0.5 McFarland medium.
2) Oil treatmentEOs were serially diluted in NB to two final concentrations: 0.5% (v/v) and 1% (v/v).
Bacterial suspensions were dispensed into 96-well plates (100 µL/well), and 100 µL of an EO 0.5% (v/v) or 1% (v/v) EO was also dispensed in each well. The control group had 8 µg/mL ampicillin (Kisan Bio, Korea) added (instead of any EO) according to a sensitivity criterion. The cells were incubated for 18 h at 37℃.
3) Bacterial growth determinationOptical density (OD) was measured at 600 nm as an endpoint using a spectrophotometer absorption program for the FlexStation 3 reader. Accuracy was improved with three replicates.
This study was conducted with the approval of the Ethics Committee of Dankook University (IRB File No. NON2021-002). The study was also conducted in accordance with the tenets of the Declaration of Helsinki.
Results1. Evaluation of the antibacterial activity of 17 EOs in comparison with an antibioticCompared with the effect of ampicillin on E. coli, TM showed the same antibacterial activity and PR and RM showed a concentration-dependent antibacterial activity similar to that of ampicillin (Figure 1).
LM, LV, RW, LG, and MS inhibited E. coli growth, mainly at 1% concentrations, but were less effective than ampicillin. All other EOs showed no antibacterial effect; some might even stimulate bacterial growth.
2.Evaluation of the antibacterial activity of 17 EOs at different concentrationsAfter comparing the activities of the EOs tested at concentrations of 0.5% (v/v) and 1% (v/v), it was observed that TM had the highest antibacterial activity, whereas MS, RM, PR, LG, and LM showed a slightly lower antibacterial activity, and PC, PM, and EC showed an even lower antibacterial activity (Table 2).
Our results also showed that TM, MS, RM, PR, and LG had high antibacterial activity at all concentrations, and EOs except for TT and BG showed a concentration-dependent increase in their antibacterial activities (Figure 2). That is, as their concentration increased, the antibacterial activity of MS, RM, PR, LV, LM, PM, and EC also increased.
3. Evaluation of antibacterial activity of EOs according to the extraction siteThe TM, MS, RM, PR, and LG extracts used here were extracted from leaves; they all showed a high antibacterial activity against E. coli. Therefore, EO antibacterial activity might be related to the extraction site. As previously mentioned, those EOs that had an antibacterial activity similar to that of the antibiotic ampicillin, i.e., TM, PR, and RM, were leaf extracts.
DiscussionIn this study, we compared the antibacterial activity of 17 EOs with that of ampicillin against E. coli. Our results showed that TM, PR, and RM had the same antibacterial activity as ampicillin. Moreover, TM had the same antibacterial activity as ampicillin at both 0.5% and 1%. The antibacterial activity of TM may be high due to the presence of carvacrol, γ-terpinene (Alsaraf et al., 2020), and thymol (Kwon et al., 2018) components. It has previously been shown that TM extracted from native plants from Oman has a high content of carvacrol, a compound that bears a higher antibacterial activity than ampicillin (Alsaraf et al., 2020). Our results are in accordance with those from many previous studies showing that TM exhibits an excellent antibacterial activity (Beksac et al., 2021; Sim et al., 2019). Here, it was shown that this activity was not concentration-dependent.
TM also improves atopic dermatitis by inhibiting the activity of Staphylococcus aureus (Alsaraf et al., 2020; Kwon et al., 2018), which may be useful for future antibacterial evaluation of this EO against other bacteria associated with atopic dermatitis.
When the antibacterial activity of EOs was separately evaluated at different concentrations, here and in previous studies, it was observed that TM, PR (da Rocha Neto et al., 2019; Oliveira Ribero et al., 2020), RM (Jawad et al., 2018), LG (Yoon & Park, 2018), and MS (Abdellatif et al., 2021) had high antibacterial activity, that LV and RW had a concentration-dependent antibacterial activity, and that TT did not have any antibacterial activity (Yi & Bu, 2017).
Previous studies have shown that EOs have high antibacterial activity (Puškárová et al., 2017), but high concentrations of EOs do not necessarily have a high antibacterial activity. The concentrations of EO exhibiting an adequate antibacterial activity in some bacteria varies, and it is important to determine the appropriate concentrations and conditions for skin and clinical applications. Here, it was determined that EOs extracted from leaves had very high antibacterial activity PR, RM, MS and LG (Bajalan et al., 2017; Nguyen et al., 2018; Wang et al., 2019), including TM (Gonzalez et al., 2021; Mehrabi et al., 2021), PR, RM (Abozahra et al., 2020), MS (Keskin & Guvensen 2018), and LG (Ilango et al., 2019; Gao et al., 2020). Thought the above five types of EOs extracted from the leaves showed the highest antibacterial activity, LV (Park & Kang, 2020) and LM (Lee et al., 2020a), which were extracted from flowers and fruit skins, also showed high antibacterial activity in a concentration-dependent manner. This is because the composition of essential oils and the mechanism of antibacterial action against bacteria differ depending on the extraction site and botanical family (Kim, 2019). Future research is needed to confirm the effects of EO blending and the appropriate application method for each EO according to an individual's condition, target infection site, and bacterial species.
ConclusionsIn this study, the antibacterial activity against E. coli was evaluated for 17 different EOs. First, it was determined that an antibacterial activity similar to that of ampicillin could be found in TM, PR, and RM. Second, among all EOs, TM showed the best antibacterial activity at a concentration of 0.5% (v/v), and TM, PR, and RM showed high antibacterial activity at a 1% (v/v) concentration. Third, EOs with the highest antibacterial activities were mainly extracted from leaves (Lee & Kim, 2021; Kim et al., 2019; Lee et al., 2020c). These results indicate that several EOs have an excellent antibacterial activity.
However, while the antibacterial activity of 17 different EOs was tested, only one antibiotic was used for comparison. Comparisons with a larger group of antibiotics would be a useful complement for the results presented here. Additional studies on effective antibacterial EO concentrations, expressed as MIC, against more than one type of bacterium and resistant strains might also be a good complement for this study.
Despite this, it is meaningful that the antibacterial activity of a number of EOs was evaluated against E. coli, an indicator bacterium, and that the comparison between such antibacterial activity with that of an antibiotic had not been addressed in previous studies. Through research on the antibacterial activities and various properties of EOs, the possibility of useful applications in the field of customized cosmetics preparation and cosmetics manufacturing has been confirmed. Our results may be used as basic data for further research on EOs.
NOTESAuthor's contribution
JMP and YSY designed, performed experiments, and wrote the manuscript. YKL did the experimental design. JKK has contributed greatly to the review and writing of the manuscript.
Author details
Jung Min Park (Graduate student), Department of Public Health Science, Dankook University, 119, Dandae-ro, Dongnam-gu, Cheonan-si, Chungnam 31116, Korea; Young Sam Yuk (Lecturer), Department of Clinical Medical Science, Dankook University, 119, Dandae-ro, Dongnamgu, Cheonan-si, Chungnam 31116, Korea; Young Ki Lee (Professor) and Jae Kyung Kim (Professor), Department of Biomedical Laboratory Science, Dankook University, 119, Dandae-ro, Dongnam-gu, Cheonan-si, Chungnam 31116, Korea.
Figure 1.Antibacterial activity of different essential oils and ampicillin.The bars show the antibacterial activity of 17 EOs and ampicillin expressed as the optical density of bacterial cultures. Among the EOs, only TM, PR, and RM showed the same antibacterial activity as ampicillin. The horizontal line marks the results obtained with ampicillin, as a reference. EO, essential oil; TM, thyme white; PR, palmarosa; RM, rosemary verbenone; MS, melisa true; LG, lemongrass; RW, rosewood; LV, lavender Bulgarian; LM, Lemon; CP, Cypress; CW, cedarwood atias; TT, tea tree; BG, bergamot; OR, orange sweet; SW, sandalwood; PC, pachuli; PM, peppermint premium; EC, eucalyptus bluegum.

Figure 2.OD differences among bacterial cultures in the presence of two different EO concentrations.Bars represent the differences in OD between cultures grown with the same EO at two different concentrations. The greater the difference in OD values, the higher the concentration-dependent antibacterial activity. EO, essential oil; TM, thyme white; PR, palmarosa; RM, rosemary verbenone; MS, melisa true; LG, lemongrass; RW, rosewood; LV, lavender Bulgarian; LM, Lemon; CP, cypress; CW, cedarwood atias; TT, tea tree; BG, bergamot; OR, orange sweet; SW, sandalwood; PC, pachuli; PM, peppermint premium; EC, eucalyptus bluegum; OD, optical density.
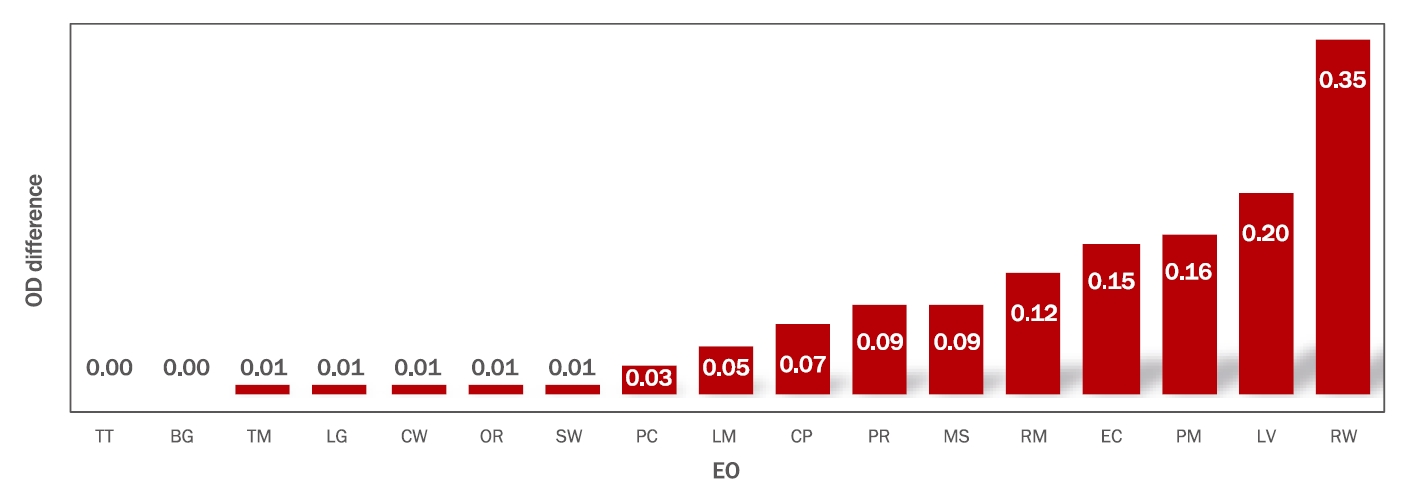
Table 1.Essential oils used in this study, and tissue/organ extraction sites Table 2.Optical density of bacterial cultures incubated with different essential oils at two different concentrations
ReferencesAbdellatif F, Akram M, Begaa S, Messaoudi M, Benarfa A, Egbuna C, Ouakouak H, Hassani A, Sawicka B, SimalGandara J, et al. Minerals, essential oils, and biological properties of Melissa officinalis L. Plants 10: 1066. 2021.
Abozahra R, Abdelhamid SM, Wen MM, Abdelwahab I, Baraka K. A nanoparticles based microbiological study on the effect of rosemary and ginger essential oils against Klebsiella pneumoniae. The Open Microbiology Journal 14: 205-212. 2020.
Ali B, Al-Wabel NA, Shams S, Ahamad A, Khan SA, Anwar F. Essential oils used in aromatherapy: a systemic review. Asian Pacific Journal of Tropical Biomedicine 5: 601-611. 2015.
Alsaraf S, Hadi Z, Al-Lawati WM, Al Lawati AA, Khan SA. Chemical composition, in vitro antibacterial and antioxidant potential of Omani thyme essential oil along with in silico studies of its major constituent. Journal of King Saud University-Science 32: 1021-1028. 2020.
Bajalan I, Rouzbahani R, Pirbalouti AG, Maggi F. Antioxidant and antibacterial activities of the essential oils obtained from seven Iranian populations of Rosmarinus officinalis. Industrial Crops and Products 107: 305-311. 2017.
Beksac K, Sahal G, Donmez HG. Thyme essential oil as an antimicrobial and biofilm inhibitory agent against abscesses with P. mirabilis Infections. Journal of Herbal Medicine 28: 100446. 2021.
Bondarenko OM, Sihtmäe M, Kuzmičiova J, Ragelienė L, Kahru A, Daugelavičius R. Plasma membrane is the target of rapid antibacterial action of silver nanoparticles in Escherichia coli and Pseudomonas aeruginosa. International Journal of Nanomedicine 13: 6779. 2018.
Croxen MA, Law RJ, Scholz R, Keeney KM, Wlodarska M, Finlay BB. Recent advances in understanding enteric pathogenic Escherichia coli. Clinical Microbiology Reviews 26: 822-880. 2013.
da Rocha Neto AC, Navarro BB, Canton L, Maraschin M, Di Piero RM. Antifungal activity of palmarosa (Cymbopogon martinii), tea tree (Melaleuca alternifolia) and star anise (Illicium verum) essential oils against Penicillium expansum and their mechanisms of action. LWT 105: 385-392. 2019.
Gao S, Liu G, Li J, Chen J, Li L, Li Z, Zhang X, Zhang S, Thome RF, Zhang S. Antimicrobial activity of lemongrass essential oil (Cymbopogon flexuosus) and its active component citral against dual-species biofilms of Staphylococcus aureus and Candida species. Frontiers in Cellular and Infection Microbiology 10: 603858. 2020.
Gonzalez K, Johnson A, Gonsalves V, Santos A. The effect of thyme essential oil on Escherichia coli. The FASEB Journal 35: 2021.
Ilango P, Suresh V, Vummidi AV, Ravel V, Chandran V, Mahalingam A, Reddy VK. Evaluation of antibacterial activity of lemongrass oil against oral clinical isolates: an in vitro study. Pharmacognosy Journal 11: 1023-1028. 2019.
Jawad AM, Allawi AK, Ewadh HM. Essential oils of rosemary as antimicrobial agent against three types of bacteria. Medical Journal of Babylon 15: 53. 2018.
Jo SJ, Park MJ, Guo RH, Park JU, Yang JY, Kim JW, Lee SS, Kim YR. Antioxidant, antibacterial, antifungal, and antiinflammatory effects of 15 tree essential oils. Korean Journal of Food Science and Technology 50: 535-542. 2015.
Keskin D, Guvensen NC. Comparative analyses of phytochemical composition and antimicrobial properties of different solvent extracts of Melissa officinalis leaves. Journal of Environmental Biology 39: 633-638. 2018.
Kim BA. Development of cosmetics preservatives using natural Essential Oil. The Journal of the Convergence on Culture Technology 5: 445-450. 2019a.
Kim HW, Kim DS, Sung NY, Han IJ, Lee BS, Park SY, Eom J, Suh JY, Park JH, Yu AR, et al. Development of functional cosmetic material using a combination of Hippophae rhamnoides fruit, Rubus fruticosus Leaf and Perillae folium leaf extracts. Asian Journal of Beauty and Cosmetology 17: 477-88. 2019b.
Kwon HI, Jeong NH, Jun SH, Son JH, Kim S, Jeon H, Kang SC, Kim SH, Lee JC. Thymol attenuates the worsening of atopic dermatitis induced by Staphylococcus aureus membrane vesicles. International Immunopharmacology 59: 301-309. 2018.
Lee JH, Kim YN, Kim DC, Chae HJ. Antimicrobial activities of edible plant extracts against oral bacteria. Journal of Applied Biological Chemistry 63: 61-67. 2020a.
Lee JY, Jhee KH, Yang SA. Antibacterial and anti-inflammatory efects of essential oil from the Magnolia kobus flower. Journal of Life Science 30: 278-284. 2020b.
Lee SM, Kim CD. Antioxidant effect of leaf, stem, and root extracts of Zingiber officinale as cosmetic materials. Asian Journal of Beauty and Cosmetology 19: 23-33. 2021c.
Lee YS, Kang YJ, Ryu MJ. Antibacterial effect and deodorization effect of extracts from different parts of Zingiber officinale. Asian Journal of Beauty and Cosmetology 18: 521-531. 2020c.
Mehrabi A, Mahmoudi R, Khedmati Morasa H, Mosavi S, Kazeminia M, Attaran Rezaei F, Shahsavari S, Vahidi R. Study of chemical composition, antibacterial and antioxidant activity of thyme leaves and stems essential oil. Journal of Medicinal Plants and By-products 2021.
Nguyen HV, Meile JC, Lebrun M, Caruso D, Chu-Ky S, Sarter S. Litsea cubeba leaf essential oil from Vietnam: chemical diversity and its impacts on antibacterial activity. Letters in Applied Microbiology 66: 207-214. 2018.
Oliveira Ribero S, Fontaine V, Mathieu V, Zhiri A, Baudoux D, Stévigny C, Souard F. Antibacterial and cytotoxic activities of ten commercially available essential oils. Antibiotics (Basel) 9: 717-733. 2020.
Park SN, Kang YJ. The effect of essential oils on antimicrobial activity. Journal of Convergence for Information Technology 10: 104-114. 2020.
Puškárová A, Bučková M, Kraková L, Pangallo D, Kozics K. The antibacterial and antifungal activity of six essential oils and their cyto/genotoxicity to human HEL 12469 cells. Scientific Reports 7: 1-11. 2017.
Sim JXF, Khazandi M, Chan WY, Trott DJ, Deo P. Antimicrobial activity of thyme oil, oregano oil, thymol and carvacrol against sensitive and resistant microbial isolates from dogs with otitis externa. Veterinary Dermatology 30: 524-e159. 2019.
Wang W, Li D, Huang X, Yang H, Qiu Z, Zou L, Liang Q, Shi Y, Wu Y, Wu S, et al. Study on antibacterial and quorumsensing inhibition activities of Cinnamomum camphora leaf essential oil. Molecules 24: 3792. 2019.
Wang X, Shen Y, Thakur K, Han J, Zhang JG, Hu F, Wei ZJ. Antibacterial activity and mechanism of ginger essential oil against Escherichia coli and Staphylococcus aureus. Molecules 25: 3955. 2020.
Wood TK. Insights on Escherichia coli biofilm formation and inhibition from whole-transcriptome profiling. Environmental Microbiology 11: 1-15. 2009.
Wu K, Lin Y, Chai X, Duan X, Zhao X, Chun C. Mechanisms of vapor-phase antibacterial action of essential oil from Cinnamomum camphora var. linaloofera Fujita against Escherichia coli. Food Science & Nutrition 7: 2546-2555. 2019.
Yi MR, Bu HJ. Antioxidant, antimicrobial and melanogenesis inhibition effects of 35 species essential oil. Journal of Korean Society of Cosmetology 23: 677-687. 2017.
Yoon H, Park C. Anti-bacterial effects of basil oil on Streptococcus mutans and Porphyromonas gingivalis. The Journal of The Korean Society of Integrative Medicine 6: 131-139. 2018.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||