요약목적당 기반 구조, 즉 XAX(Xylitylglucoside-Anhydroxylitol-Xylitol)가 피부 장벽 기능과 보습에 미치는 영향을 in vitro 및 in vivo에서 조사하고 글리세린과의 효능을 비교하고자 하였다.
방법RT-qPCR 분석을 통해 인공피부모델에서의 유전자발현을 측정하였다. 크로마토그래피를 통해 국소 처리된 인간 피부 외식편에서 세라마이드 신합성을 측정하였다. 기본 세포 배양에서 필수 단백질의 생산을 모니터링하였다. 즉 크로마토그래피를 사용하여 섬유아세포의 콘드로이틴 설페이트 및 히알루론산을 측정하였고, ELISA를 사용하여 케라티노사이트의 히알루론산을 측정하였다. 피부가 건조한 25명의 여성 지원자를 대상으로 표피 미세부조, 피부 정전용량 및 경표피 수분 손실(TEWL)을 측정하였다. 그리고 하이드로알코올성 젤과 비교하여 손의 피부 정전용량과 TEWL도 측정하였다.
AbstractPurposeThe study aimed to investigate the effects of a sugar-based structure, i.e. XAX (Xylitylglucoside-Anhydroxylitol-Xylitol) on skin barrier function and moisturization, both in vitro and in vivo, and to compare its efficacy with that of glycerin.
MethodsExpression of genes was investigated by RT-qPCR analysis on topically-treated human reconstructed epidermis. Ceramide neosynthesis was measured by chromatography in topically-treated human skin explants. Production of essential proteins was monitored in primary cell cultures: Chondroitin sulfate and hyaluronic acid in fibroblasts, using chromatography; hyaluronic acid in keratinocytes, using ELISA. In vivo, epidermal microrelief, skin capacitance and transepidermal water loss (TEWL) were measured on 25 female volunteers with dry skin. Skin capacitance and TEWL were also measured, on hands, comparing hydroalcoholic gels.
ResultsXAX increased the expression of key moisturizing-related genes, such as enzymes, structural components or regulators of inter-keratinocytes junctions, cornified layer, desquamation process, as well as skin barrier function and hydration. It also increased the contents of ceramides, hyaluronic acid and chondroitin sulfate. In vivo, XAX treatment at 3% decreased TEWL and increased skin capacitance, skin microrelief and desquamation. The combination of XAX+glycerin (1.5%+1.5%) showed interesting effects compared to glycerin alone.
ConclusionXAX promoted effects on the biological pathways involved in skin barrier function; epidermal and dermal water reserves; and epidermal water circulation. Its effectiveness, confirmed in vivo, alone and in combination with glycerin, makes it an interesting alternative to basic emollients, humectants and occlusives.
中文摘要方法 通过 RT-qPCR 分析对局部处理的人类重建表皮进行基因表达研究。在局部处理的人皮肤外植体中通过色谱法测量神经酰胺新合成。在原代细胞培养物中监测必需蛋白质的产生:使用色谱法测定成纤维细胞中监测硫酸软骨素和透明质酸; 使用ELISA角质形成细胞中的透明质酸。对25名皮肤干燥的女性志愿者测量了表皮微起伏、皮肤电容和经表皮水分流失 (TEWL)。还测量了手上的皮肤电容和TEWL,并比较了醇凝胶。
IntroductionSkin is a complex organ that covers the whole body. Some of its main functions are to protect the body from environmental aggressions and to contribute to its homeostasis. Skin moisturization is key for the outward appearance of healthy skin (Draelos, 2018). For instance, dryness is characterized by roughness, a papyraceous appearance of the surface, the presence of raised squames and/or scales and irritation (Haftek, 2015). Epidermis, the most superficial tissue of the skin, contributes greatly to the barrier function of the skin. This pluristratified tissue, which is continuously self-renewing, is mainly composed of keratinocytes (85-95%). Keratinocytes pass through a tightly regulated differentiation program and sequentially form stratum basale (SB), stratum spinosum (SS), stratum granulosum (SG), and stratum corneum (SC) layers of the skin (Goleva et al., 2019; Lefèvre-Utile et al., 2021). Correct keratinocyte differentiation and epidermal homeostasis are required to ensure an appropriate state of skin hydration.
Each epidermal layer contains keratinocytes at a particular stage of differentiation and is defined by its expression of characteristic morphological and biochemical features, aiming to produce a cohesive tissue. For instance, basal keratinocytes have high proliferative capacity and are key for epidermal maintenance and regeneration. Expressions of keratins (KRT) 5 and 14 are characteristic of these cells. Moreover, a 2-Mb locus on the human chromosome 1 called the epidermal differentiation complex (EDC) comprises sixty genes coding for proteins involved in keratinocyte differentiation and SC properties (Henry et al., 2012). Among these proteins, repetin (RPTN) is detected closely associated with granular keratinocytes as well as loricrin (LOR) and filaggrin (FLG). Involucrin (IVL) and LOR expression especially increases with keratinocyte differentiation. Transglutaminases (TG), especially TGM1, cross-link IVL, LOR, and other structural proteins to form the cornified envelope and to provide the SC its mechanical strength (Goleva et al., 2019). Shortly before cornification, the keratinocytes synthesize and excrete corneodesmosin (CDSN), which spontaneously embeds within the SG desmosomes (Haftek, 2015). Calmodulin-like 5 (CALML5), is also essential for late epidermal differentiation (Sun et al., 2015). Finally, the cornified layer is mainly composed of two layers of LOR, the whole being surrounded by multilamellar lipid structures and by hydrophilic intracorneocyte substances, mainly the natural moisturizing factor (NMF).
The main constituents of SC lipids are ceramides (45%-50%), cholesterol (25%) and free fatty acids (10%-15%). Ceramides are essential to form a natural barrier to transepidermal water loss (Lefevre-Utile et al., 2021). At the end, to enable the desquamation process, CDSN must be degraded by proteases, together with the desmosomal cadherins. Kallikreins (KLK)5 and KLK7, especially, cleave the extracellular components of corneodesmosomes, such as CDSN, loosening the cellular connections and enabling desquamation (Haftek, 2015; Mc Govern et al., 2017).
In addition to appropriate keratinocyte differentiation and SC structuration, water transport, within the epidermis, can be mainly regulated by both aquaporins (AQP) and tight junctions (TJ). TJ are detected in the granular layer and constitute cell-cell seals (Zaniboni et al., 2016). Among these proteins, Claudins (CLDN), occludin, zonula occludens (ZO), cingulin (CGN) and Multi-PDZ Domain Protein 1 (MUPP-1) are associated with the functional TJ barrier (Crawford & Dagnino, 2017).
The dermis is responsible for biomechanical properties of the skin, such as suppleness, has also a positive impact on the appearance of the skin surface as well as on the water content of the skin. This is mainly due to the presence of extracellular matrix (ECM) components. Among them, glycosaminoglycans (GAG), and more especially hyaluronic acid (HA), act as water reservoirs for the skin, thus participating in skin moisturization (Sodhi & Panitch, 2021). For instance, HA is able to bind up to 1000 times its weight in water. On the contrary, decrease in GAG content induces skin dryness. Similar to HA, chondroitin sulfate (CS), another GAG, when bound to a proteoglycan such as aggrecan, plays a key role in retention of water.
The analysis of the impact of an ingredient on the expression of these typical genes and proteins are of high interest to understand its mechanism of action.
The critical role of skin hydration for maintaining healthy skin explains why moisturizers are important components of basic skin care. Indeed, it is a universal need, for populations of all ages and in most geographies around the world. In this context, this study aimed to investigate both in vitro and in vivo effects of a specific sugar-based structure (liquid and water-soluble), i.e. XAX (INCI name: Xylitylglucoside-Anhydroxylitol-Xylitol), on skin barrier function and moisturization. XAX efficacy was also compared with that of glycerin in most of the in vitro models as well as in a specific clinical trial, by measuring the prevention of skin dehydration induced by hydroalcoholic gels.
Methods1. Expression of moisturizing-related genes in a 3D model of human reconstructed epidermisReconstructed epidermis (EPI/001; StratiCELL®, Belgique) were at the air-liquid interface in a proprietary serum free medium and were maintained in a humid atmosphere at 37℃ with 5% CO2. When the epidermis were fully differentiated after 14 days, either placebo oil-in-water emulsion (without XAX) or the same formulation containing 3% XAX (Table 1) were topically applied at a concentration of 2 mg/cm2.
Experiments were realized in culture duplicates. After 24 hours of incubation, total ribonucleic acids (RNA) from epidermal tissues were extracted with the RNeasy Mini Kit (Qiagen, US) following the recommendations of the supplier and reverse transcribed into complementary DNA (cDNA). The expression of 92 genes, involved in epidermal differentiation and skin barrier function, was analyzed by qPCR on each cDNA, using a dedicated microfluidic-based 384-well TaqMan array (Applied Biosystems, USA). Cycle thresholds (Ct) were calculated and gene expression analysis was performed using the Data Assist software from Applied Biosystems. Ct values were normalized with regard to a housekeeping gene (hk) and Relative Quantity (RQ) was obtained using the following calculation: 2-(△Ct XAXtreated condition - △Ct placebo-treatedreference condition), where △Ct=Ct target gene-Ct hk for each cDNA sample. Only genes presenting RQ values higher than 1.4 and a standard deviation lower than 30% were considered. Significant pathway analysis was performed with the GeneSpring® software (Agilent).
2. Measure of ceramide neosynthesis in human normal skin explantsHuman skin explants were prepared from an abdominoplasty of a 35 year-old woman. The skin discs of 8 mm of diameter were cultured in MEM/M199 (3:1, Gibco) supplemented with 50 IU/mL penicillin, 50 µg/mL streptomycin, 0.2% (w/v) sodium bicarbonate, 2% fetal calf serum (FCS; Sera Tech Zellbiologische Produkte, Skt Salvator, Germany) and 1 µCi/mL carbon 14-labeled acetate (Gibco).
Placebo cream gel or the same formulation with 3% glycerin or 3% XAX (Table 1) were applied to the surface of the skin explants, at a concentration of 2 mg/cm2. epidermal growth factor (EGF) 10 ng/mL was tested as reference molecule in skin explant medium. Control skin explants were also incubated in the absence of product. The skin explants were incubated at 37following calculation: - 5% CO2 for 18 hours (triplicate). At the end of incubation, the epidermis was separated from the dermis by controlled thermal shock (MilliQ water, 2 min, 62℃) and digested with trypsin 1% (ICN Biomedicals Inc., USA) overnight at 37℃. The neosynthetized lipids, labeled with carbon 14, were extracted by partition between an organic phase (methanol/chloroform (1:2) and an aqueous phase (0.25 M potassium chloride). The organic phase was then evaporated under nitrogen and the residues were taken up in a (2:1) chloroform/methanol mixture. Samples (20 µL, corresponding to 3700 counts per minute, cpm) were then applied to silica 60 chromatography plates (Merck, Allemagne) and eluted in three successive solvents: chloroform/acetone/methanol (38:2:10); chloroform/acetone/methanol (40:5:5); chloroform/ethyl acetate/ether/methanol, (36:10:3:1). Ceramides 1 and 2 position was distinguished from that of the other epidermal lipids using appropriate standards. The radioactivity of the separated spots was counted using a radioactivity analyzer (Storm, UK). The results were expressed in cpm/mg of epidermis. For each treatment group, the mean and the SD were calculated. The treated groups were compared with the control group by a Student's t-test (p<0.05).
3. Measure of hyaluronic acid content in normal human keratinocytesNormal human epidermal keratinocytes (NHEK, from a female donor of 37-years old; Lonza, Bâle, Switzerland) were cultured in 48-multiwell plates in Keratinocyte Growth Medium (KGM) Gold (Lonza), for 5 days at 37℃ in a humidified atmosphere with 5% CO2. Then, NHEK treated (or not) with 0.01% or 0.1% XAX, or the reference molecule, i.e. 0.2 µM retinoic acid (Sigma-Aldrich) (quadruplicate treatment). After a 24 h-incubation period, 150 µL of supernatants of each well were collected for the measurement of HA. At the end of the incubation (48 h), cells were lyzed with a RIPA buffer to quantify total proteins.
The content of HA in the supernatants was measured with a specific enzyme-linked immunosorbent assay (ELISA) kit (Hyaluronan DuoSet kit, R&D Systems, Minneapolis, USA. The total protein content in the cell lysates was quantified using the bicinchoninic acid assay (Interchim, France). For each group, the mean and the SD of the normalized HA quantities (ng HA/µg total proteins) were calculated, as well as the percentages of variation in comparison with the control group (untreated cells). Statistical significance was assessed using Student's t-test with p<0.05 being considered significant.
4. Measure of hyaluronic acid and chondroitin sulfate contents in normal human dermal fibroblastsNormal human dermal fibroblasts (NHDF, from a 36 year-old female donor; Biopredic International, France) were cultured in MEM/M199 (3:1, Gibko) supplemented with 2 mM L-glutamine, 50 IU/mL penicillin, 50 µg/mL streptomycin, 0.185% (v/v) sodium bicarbonate and 10% FCS at 37℃ in a humidified atmosphere with 5% CO2. After 13 days, cells were treated not with either XAX (at 0.001%, 0.01% or 0.1%), glycerin (at 0.01% or 0.1%) or the reference molecule, i.e. 10 ng/mL EGF (triplicate). After 4 days of incubation, the supernatants were collected for the dosage of hyaluronic acid (HA) and chondroitin sulfate (CS). Their quantification was done by spectrophotometry at 630 nm and 450 nm, respectively, after staining with STAINS ALL (Sigma-Aldrich, USA). Results were expressed in µg/mL HA or CS. For each treatment group, the mean and the standard deviation (SD) were calculated, as well as the percentages of variation in comparison with controls (untreated cells). The treated groups were compared with the control group by a Student's t-test (p<0.05).
5. Design of the clinical trial on body skin hydration comparing the emulsion with 3% XAX to the placebo emulsionA randomized double blind clinical study was conducted under medical control on 25 female volunteers (mean age: 40 years-old, from 22 to 60 years-old) with dry skin on legs (corneometry value between 45±5 % and 55±5 % arbitrary units (a.u.)).
On the first day of the study (D1), placebo Oil-in-Water emulsion and the same formulation with addition of 3% XAX (Table 1) was applied on the outer part of one leg at a dose of 2 mg/cm2. An area to which no product was applied was used as an "absolute control" area to follow the variability of measures on the first day: D1T0 before any application of product and D1T8h 8 hours after the first application of product. The volunteers subsequently used the creams on their legs (one per leg; randomized) twice daily from D1 evening to D29 evening. Intermediate and final examinations were done after 15 days (D15) and 30 days (D30) of application. Each time, the volunteers arrived at the assessment center without having applied any cream (the last application had to be approximately 12 hours earlier). All the measurements were made in an air-conditioned room (18±1℃, relative humidity 55%±5%), after acclimation of the volunteers for at least 30 minutes.
1) Microrelief and desquamation evaluationAt each time of measurement (D1T0, D15 and D30) one SC sample was taken from an area of the outer part of the legs using a Diagnoskin®. The controlled-adhesiveness transparent square was applied at constant pressure (about 150 g/cm2 for 10 s) to the measurement area, then peeled off. Morphological analysis of microdepressionary network (skin microrelief) and desquamation were evaluated by microscopical examination and quantified by validated scoring (score from 1 to 12). Means, SD and SEM were calculated for each measurement time, as well as percentages of variations between Placebo-mean values and XAX-mean values.
2) Measure of skin capacitanceSkin hydration was measured at D1T0, D1T8h, D15 and D30. Each time, three measurements were made on the outer part of each leg using a CM 820TM corneometer (Courage and Khazaka Electronic Gmbh., Germany) and mean values were considered. The moisture content in the SC was calculated according to the following equation: Z={R2+[(1/(2πfC)]2}½, with Z: impedance; R: resistance; f: current frequency, C: capacity. Obtained values were expressed as a.u (Jemec & Na, 2002).
3) Measure of TEWLA TEWL measurement was performed at D1T0, D1T8h, D15 and D30. TEWL was measured on the outer part of each leg using an evaporimeter (Tewameter TM 210; Courage and Khazaka Electronic Gmbh). Results were expressed in g/m2/h.
4) Data treatmentFor each product, means and SEM were calculated for each measurement time and point (corneometry and TEWL). Percentage of variation between placebo and XAX at the different times were calculated, according to the following formulas:
%=(TAtiXAX - TAtiplacebo)/ TAtiplacebo
with TA: mean value obtained on the treated zone / ti: at different measurement times after product application).
For skin capacitance, intra-group and inter-group comparisons were performed on values at each time of measurement using a Student's t-test, with a 5% significance level.
For TEWL, intra-group and inter-group comparisons were performed on values at each time of measurement and changes over time respectively using a Student's t-test.
6.Design of the clinical trial on hand skin hydration, comparing hydroalcoholic gels with either an association of 1.5% XAX+1.5% glycerin or 3% glycerin aloneA clinical study was conducted under medical control on 3 groups of 20 female volunteers presenting all skin types (aged from 20 to 63 years-old, mean age between 41 and 48 yearsold depending on the group). A wash-out period of 48 hours preceded the trial. Then, the volunteers had to apply the placebo hydroalcoholic gel and the same formulation with addition of 3% XAX or addition of 1.5% XAX+ 1.5% glycerin (Table 1) at least 5 times per day (mean number of applications: 8 per day), with appropriate instructions of use.
1) Measure of skin capacitanceThe measurement of the skin moisturization was performed using a corneometer CM825® (Courage & Khazaka, Electronic GmbH., Cologne, Germany) at T0 before any product application and after 24 h of product use.
2) Measure of TEWLTEWL measurement was performed using a Tewameter 300® (Courage and Khazaka, Electronic GmbH) at T0 before any product application and 8 days of product use.
3) Self-assessmentAt the end of the study volunteers' opinion on the tested products was investigated by means of a self-assessment questionnaire.
4) Data treatmentFor each product, mean, SD and SEM were calculated for each measurement time and point (corneometry and TEWL). Percentages of variations versus T0 were calculated, according to the mean of the percentages of variation for each volunteer [(TAti-TAt0)/TAt0]×100. Percentages of variation versus placebo were calculated according to the formula:
{[(TAti-TAt0)XAX-(TAti-TAt0)placebo]/[TAt0XAX+(TAti-TAt0)placebo]}×100
(where: TA: mean value obtained on the treated zone / t0: before product application/ti: at different measurement times after product application).
The results of self-assessment questionnaires were calculated as percentages (%) of subjects who assigned a particular judgment (among those proposed). The instrumental data were submitted to Student's t-test. The intra-group statistical analysis was carried out on raw data versus baseline (T0). The inter-group statistical analysis was carried out comparing the variation recorded for each active product versus the corresponding placebo product at each experimental time. Variations were considered statistically significant when p value was<0.05.
Results1. Expression of genes involved in skin hydration in a human reconstructed epidermisFold-change and pathway analyses performed on results from RT-qPCR investigations showed that, in comparison with placebo treatment, the topical application of the XAX-containing formulation on 3D reconstructed human epidermis induced an upregulation of genes encoding proteins involved in skin barrier function, skin water reserves and epidermal water circulation (Table 2). During the experiments, it was also observed an increased expression of aquaporin 3 but the level did not reach the threshold (i.e. 1.32; data not shown).
2. Neosynthesis of ceramides in a human skin explant modelA model of skin explant was used to investigate the effect of XAX on the neoproduction of ceramides.
In our experimental conditions, EGF, tested at 10 ng/mL in skin explant medium, increased neosynthesis of epidermal ceramides 1 and 2 by 61% in comparison with untreated explants (p<0.05; Figure 1). XAX formulated at a concentration of 3% and applied to the surface of skin explants, increased neosynthesis of epidermal ceramides 1 and 2 by 139% in comparison with untreated explants (p<0.05; Figure 1). The increase of 73% which was observed for the placebo formulation was not statistically significant compared to untreated explants (Figure 1). Glycerin, formulated at 3%, had no significant effect on neosynthesis of epidermal ceramides 1 and 2 in comparison with untreated explants (+28%; Figure 1).
3. Assessment of key proteins produced by fibroblasts and keratinocytesEffects of XAX or glycerin were investigated using primary cell cultures.
1) Production of hyaluronic acid and chondroitin sulfate in normal human dermal fibroblasts culturesEGF, tested at 10 ng/mL in the culture medium, increased the extracellular HA content by 47% in comparison with untreated cells (p<0.01; Figure 2A). XAX, tested at 0.01% and 0.1% increased the extracellular HA content by 20% (p<0.05) and 19% (p<0.1) respectively, in comparison with untreated cells, while the lowest concentration, i.e. 0.001%, showed no significant effect (Figure 2A). Moreover, XAX, when tested at 0.01%, showed a statistically significant promoting effect in comparison with glycerin 0.01% (+20%; p<0.05), as well as in comparison with glycerin 0.1% (+19%; p=0.05; Figure 2A). Glycerin, which was also tested at 0.01% and 0.1%, had no effect on the extracellular hyaluronic acid content (Figure 2A).
Looking at CS contents, EGF, tested at 10 ng/mL, increased by 45% the extracellular content in CS (Figure 2B; no significant effect in comparison with untreated cells). XAX, tested at 0.001%; 0.01% and 0.1%, increased the extracellular content in CS in comparison with untreated cells, with a dose-dependent effect (+80%, +166% and +233% respectively). These increases were statistically significant for the two highest concentrations of XAX (p<0.05) (Figure 2B). XAX, tested at 0.1% also increased the extracellular content in CS, in comparison with glycerin 0.1%-treated cells (+287%, p<0.05; Figure 2B). Glycerin, tested at 0.01% and 0.1%, had no significant effect on extracellular content in CS (Figure 2B).
4. Effects on skin hydration in vivo1) Effect on the body when used in a conventional body care emulsionXAX improved epidermal microrelief and increased skin capacitance, while decreasing transepidermal water loss (TEWL). XAX induced an improvement of both skin microrelief and desquamation, of +33% (p<0.1) (Figure 3A) and +25% (p<0.1 versus placebo respectively; Figure 3B), after 30 days of treatment. The effects could be clearly visible on microscopic observations as illustrated by typical photos of one of the volunteers (Figures 3A and 3B; right part).
A statistically significant increase in skin hydration (corneometry) at 8 h, 15 days and 30 days after treatment was also demonstrated (of +6%, +5% and +11% respectively; Figure 3C). Finally, XAX also improved skin barrier function (decrease in TEWL) at 8 h, 15 days and 30 days after treatment (of -8% at 8 h and 15 days, -15% at 30 days; Figure 3D). This effect was statistically significant at the end of the treatment.
2) Effects on hands when used in a hydroalcoholic gelThe formula containing 1.5% of XAX+1.5% of glycerin had a significant short-term moisturizing effect with an increase in skin capacitance of +13.5% after 24 hours versus D0 (p<0.01; Figure 4A) and +13.8 % versus placebo. 90% of the volunteers felt their hands moisturized and 95% felt their skin more comfortable. The formula containing 3% of glycerin also showed an immediate significant moisturizing effect with increased skin capacitance by +9.8% versus D0 (p<0.01; Figure 4A) and by +11.2% versus placebo. As with the previous formula, 90% of the volunteers felt their hands moisturized and 95% felt their skin more comfortable. In the meantime, no improvement was observed for the placebo group regarding the skin moisturization (Figure 4A).
The formula containing 1.5% of XAX+1.5% of glycerin also improved skin barrier function in the long term as showed by a significant decrease in the transepidermal water loss by -15.4% after 8 days versus D0 (p<0.01; Figure 4B), and by -11.2% versus placebo. 95% of the volunteers felt their hands moisturized and 100% reported a sensation of a more comfortable skin. The formula containing 3% of glycerin also showed a long term hydration effect by significantly decreasing the transepidermal water loss by -7.8% after 8 days versus D0 (p<0.05; Figure 4B), but had no significant effect versus placebo (-3.2%). 95% of the volunteers felt their hands moisturized and 100% felt their skin more comfortable. In the meantime, no improvement was observed for the placebo group regarding the skin barrier function (Figure 4B).
DiscussionSkin barrier function is vital for human survival, and skin hydration is critical for maintaining healthy skin. Therefore, moisturizers are the most used skin care ingredients, often prescribed by dermatologists. Most of them act as emollients, humectants (such as glycerin) or occlusives, while others are known to imitate or to promote the production of skin natural components involved in hydration, such as hyaluronic acid (HA). In the present study, the effects of a specific sugar-based structure, i.e. XAX (INCI name: Xylitylglucoside-AnhydroxylitolXylitol), were investigated on skin barrier function and moisturization, both in vitro and in vivo. Robust results have been observed in vivo, with or without glycerin, in aqueous and hydroalcoholic formulas, with multiple measurement parameters, and supported by a complete biological mode of action.
1. Mode of action1) Promotion of skin barrier functionFirst, the results showed that XAX promotes the neosynthesis of epidermal ceramides in a human skin explant model, when topically applied, with a better efficacy than the placebo formulation. SC ceramides are the major constituents of SC lipids. These lipidic components endow the cornified layer with its water-repellent properties and are strongly connected via corneodesmosomes, ensuring the physical strength and the mechanical resistance of the cornified envelope. In addition to the free lipids of the SC matrix, there are ceramides covalently bonded to the corneocyte protein envelope. A decrease in ceramide contents is observed in dry skins, such as atopic skins (Hogan et al., 2012). Thus, the promoting effects of XAX on SC ceramides production certainly contribute to its observed in vivo moisturizing effects. XAX also induced, in a reconstructed epidermis model, an increase in gene expression of 3-hydroxy3-methy-lglutaryl-CoA reductase (HMGCR), a key enzyme in the cholesterol biosynthetic pathway (Faulkner & Jo, 2022). Since ceramides and cholesterol represent the major components of SC lipids, the ability of XAX to prevent TEWL in several clinical trials is certainly correlated to its promoting effects on these two categories of epidermal lipids. From these first results, it can be concluded that XAX can regulate the lipid composition of SC and of epidermis, thus probably participating in the reinforcement of skin barrier function.
Moreover, XAX effects on the expression of genes, whose corresponding encoded proteins are enzymes or structural components of the cornified layer, as well as regulators of the desquamation process, are likely to participate further in the increase in skin barrier function observed in vivo. Indeed, as assessed by RT-qPCR investigations on topically-treated human reconstructed epidermis, the XAX-regulated genes calml5, cdsn, ivl, lor, rptn, tgm-1 and -5, as well as klk5 and klk7. ivl, lor and rptn genes belong to the Epidermal Differentiation Complex (EDC), involved in the formation of the cornified envelope. More particularly, transglutaminase-1 (TGM1) cross-links IVL, LOR, and other structural proteins, in order to form the cornified envelope and to provide the SC its mechanical strength. Shortly before cornification, the keratinocytes of the granular layer synthesize and excrete into the extracellular spaces a glycoprotein, CDSN, which spontaneously embeds within the intercellular portions of the SG desmosomes occupied by cadherins (Haftek, 2015). During the desquamation process, CDSN must be degraded by proteases, together with the desmosomal cadherins. A complex interplay of serine proteases (kallikreins, KLK) and cysteine proteases (cathepsins), with their respective inhibitors, is orchestrated by the modifications of SC pH and hydration to result in the progressive digestion of the corneodesmosomes (Haftek, 2015; Mc Govern et al., 2017).
Taken together, these results confirm that XAX regulates several genes and support the concept that it exerts a wide panel of biological effects, concurring to the production of main proteins and lipidic components of the epidermis, themselves known as being critical for skin hydration.
2) Promotion of skin water reservesIn addition to its effects on biological targets involved in SC structure and skin barrier function, XAX could induce an increase in the contents of HA in both normal human dermal fibroblasts and normal human keratinocytes. In tubo enzymatic assay also highlighted that XAX, tested at 0.01% and 0.05% significantly inhibited hyaluronidase activity in comparison with control conditions, while glycerine tested in the same conditions and at the same doses, had no effect (data not shown). In addition, XAX-treated normal human dermal fibroblasts showed increased productions of CS. The presence of the ionizable groups (sulfates and carboxylates on hexuronic acids) confers GAGs their key abilities such as water retention (Sodhi & Panitch, 2021). Similar to HA, CS promotes water retention, when it is bound to a proteoglycan, such as aggrecan.
At the epidermal level, natural moisturizing factors (NMF) act as water reservoirs. Indeed, their main function is actually not to increase the water content, but rather to replace water in dehydrated conditions and thereby retain fluidity in SC lipid and protein components (Mojumdar et al., 2017). Amino acids of the NMF partially come from the degradation of FLG. More precisely, Caspase-14 (CASP14) is involved in the cleavage of profilaggrin into FLG units, that can be integrated in keratin intermediate filaments (KIF) to stabilize and change the shape of the cells. This crosslinking reaction is also catalysed by transglutaminases, which participates in the fine-tuning of the correct maturation and function of the cornified envelope (Goleva et al., 2019).
Thus, XAX could regulate the expression of both epidermal and dermal components involved in skin water reserves.
3) Promotion of water circulationWithin the epidermis, tight junctions (TJ) acts on water transport. TJ constitute cell-cell seals. In normal human skin, they are essential for cell differentiation and keratinization of epidermal cells. TJ play an important role in epidermal selective permeability, controlling intercellular flow of substances. Claudins (CLDN), occludin, zonula occludens (ZO), cingulin (CGN) and Multi-PDZ Domain Protein 1 (MUPP-1) localize to cell-cell borders in the SG, and are associated with the barrier function of TJ (Crawford & Dagnino, 2017).
RT-qPCR experiments on topically-treated reconstructed human epidermis showed that XAX was able to increase the expression level of several genes encoding TJ, especially cgn, cldn-4, cldn-5 and cldn-7. CGN is a peripheral membrane protein, which binds to ZO-1 at tight junction plaques. This protein is expressed within the SG (Shi et al., 2018) and plays both structural and signaling roles (Crawford & Dagnino, 2017). The anchoring and organization of microtubules mediated by CGN requires phosphorylation of this protein at TJ sites, further illustrating the importance that CGN has as a signaling hub. CLDN, for their part, belong to a superfamily currently composed of 27 small transmembrane proteins in humans/mammals. They regulate conductance through the paracellular pathway by size and charge selection. Individual claudin proteins are generally classified as either barrier-forming or pore-forming (channelforming) claudins based on whether their expression increases or decreases permeability (Shi et al., 2018). For instance, CLDN1 and 4 are classified as barrier CLDN (Shi et al., 2018). In these same RT-qPCR experiments, the gene expression level of tlr2 was also increased by XAX treatment. Different studies report a link between Toll-like receptors (TLR) and TJ functions. TLR are well-known transmembrane proteins that play as innate receptors. Epidermal keratinocytes express several TLR, located either on the cell surface (TLR1, TLR2, TLR4, TLR5 and TLR6) or in endosomes (TLR3, TLR7 and TLR9) (Sun et al., 2019). TLR2 activation enhances skin barrier in human skin and is an important part of a wound repair response (Kuo et al., 2013). On the contrary, reduced epidermal TLR2 expression observed in atopic patients may play a role in their incompetent skin barrier (Kuo et al., 2013) and there are reports on genetic variants of TLRs that are associated with this disease (Zaniboni et al., 2016). Moreover, activation of TLR2 can regulate expression of TJ proteins (Zaniboni et al., 2016; Kuo et al., 2013). Thus, taken together, these results suggest that XAX could regulate TJ functions, by promoting the expression of their key components, as well as TLR2-induced related regulating mechanisms.
Thus, acting on TJ and TLR2 expression, XAX appears as a regulator of biological pathways which are involved in paracellular water circulation.
The biological effects of XAX on the production of skin water reserves in both epidermis and dermis and strengthening of skin barrier is most likely at the origin of its ability to promote skin capacitance, while reducing TEWL. Indeed, our body is composed of approximately 70% water, with 20% of this amount being accumulated in the skin. In this specific interfacial tissue, 60-70% of this amount is located within the dermis (Guzman-Alonso & Cortazar, 2016) and the epidermis absorbs the water from the dermis. The mechanical properties of the skin, in terms of strength and elasticity, are known to be affected by hydration, and the water content in SC is indeed the primary factor that governs its flexibility (Mojumdar et al., 2017). Thus, the benefits observed on skin microrelief, as well as on skin surface aspect, are also certainly due to the global biological action of XAX on SC function, water reserves and circulation.
2. Promotion of skin hydration in vivoAs mentioned before, most moisturizing products on the market use emollients, humectants and ingredients with occlusive properties. Glycerin is an ingredient having hygroscopic properties: it "captures" water molecules. As such, it is used widely in cosmetic products all over the world. Considering the wide use of glycerin and the recent pandemic situation resulting in extensive use of hand disinfectant gels containing high concentrations of alcohols (ethanol, isopropanol), it appeared of interest to evaluate the benefit of XAX in this very challenging hydroalcoholic formulation associated with glycerin. The formula containing 1.5% of XAX+1.5% of glycerin showed an immediate moisturizing effect by significantly increasing the skin capacitance after 24 hours, and a long-term effect, with a significantly decreasing TEWL after 8 days, in comparison with placebo, and with a greater effect than the formula containing 3% of glycerin alone. Moreover, the high level of volunteers' satisfaction regarding moisturizing feeling and comfort, which was the same for the two formulations. These results showed that XAX can provide hydroalcoholic gels protective benefits in terms of skin barrier function, hydration and comfort. The aforementioned biological effects could explain XAX better moisturizing efficacy than the hygroscopic properties of glycerin. Indeed, all along the different in vitro experimentations, the absence of effects of glycerin on the following biological targets was confirmed: neosynthesis of epidermal ceramides, extracellular hyaluronic acid and chondroitin sulfate contents in fibroblasts, and hyaluronidase activity (data not shown). In the future, synergistic effects between XAX and glycerin could be further investigated and compared at both biological and chemical levels. It would be of great interest to compare the interactions of XAX with glycerin on full-genome expression on topically-treated explants or reconstructed epidermis.
ConclusionIn conclusion, XAX demonstrated its benefits in terms of skin moisturization. First, in vitro, it could act on the three main biological pathways involved in epidermal and dermal hydration (Figure 5):
1- Epidermal differentiation and skin barrier function;
2- Skin water reserves (within both the epidermis and the dermis), and
3- Paracellular water circulation within the epidermis.
Second, in vivo, its efficacy on skin moisturization and protection was proven at the dose of 3% in a standard moisturizing protocol and routine on dry skins. Interesting effects were also observed when used in stringent conditions of use of a hydroalcoholic gel, in combination with glycerin formulated at two times less.
Taken together, these results confirm and reinforce XAX as a powerful moisturizing cosmetic ingredient, proposing alternatives or interesting effects in combination with basic emollients, humectants and occlusives.
NOTESAuthor's contribution
C.G. conceptualized, supervised, administered the project (including the design of all experimental investigations), and reviewed the manuscript. E.H. administered, collected and analyzed the data of clinical studies. E.V. contributed to formal data analysis and writing of the original draft. C.K. validated the formal analysis. C.K. and A.R. contributed equally to manuscript edition and revisions. A.R. visualized the data.
Author details
Christine Garcia (Director of Applied Research, PhD)/ Elodie Valin (Senior Researcher, PhD)/Elsa Hernandez (Researcher, MD)/Catherine Kern (Biological evaluation manager, PhD)/Alicia Roso (Scientific communication manager, MD), Seppic, 50 boulevard National, 92257 La Garenne Colombes Cedex, Paris, France.
Figure 1.Levels of epidermal ceramides in topically-treated skin explants.Results obtained by chromatography are expressed in cpm/mg ceramides 1 and 2 (y-axis), as well as % of increase in comparison with control cultures in brown and blue, for the comparison with EGF and 3% XAX, respectively). *p<0.05 (Student’s t-test).

Figure 2.Protein contents in primary keratinocytes and fibroblasts cells culture.(A) Levels of hyaluronic acid (HA) in NHDF cultures after 4 days of incubation. Results are expressed in μg/mL HA (y-axis); (B) Levels of chondroitin sulfate (CS) in NHDF cultures after 4 days of incubation. Results are expressed in μg/mL CS (y-axis); (C) Levels * of hyaluronic acid (HA) contents in keratinocytes. Results are expressed in ng HA/µg proteins (y-axis); The data are presented (A to C) as quantity with respectively described units as well as percentages of increase (on the top of the corresponding histograms); Student's t-test: LS (Limit of significance) p<0.1, p<0.05, **p<0.01.

Figure 3.Clinical results on body skin hydration (emulsion formulation).(A) Benefits on skin microrelief and (B) Benefits on skin desquamation; (A to B) Scorage obtained from Diagnoskin® samples observations are represented, as well as percentages of increase in comparison with placebo (in bold blue) (left part), illustrations of representative microscopic observations of Diagnoskin® samples (right part); (C) Benefits on corneometry. Results obtained after skin capacitance measures are expressed in arbitrary units (a.u.; y-axis), as well as percentages of increase in comparison with placebo (in bold blue), for each endpoint (either T0 or 8 h, 15 days or 30 days after the beginning of the treatment); (D) Benefits on TEWL. Results obtained after evaporimeter measures are expressed in g/m2/h (y-axis), as well as percentage of increase in comparison with placebo (in bold blue), 30 days after the beginning of the treatment; (A to D) Student's t-test: LS (Limit of significance) p<0.1, *p<0.05.
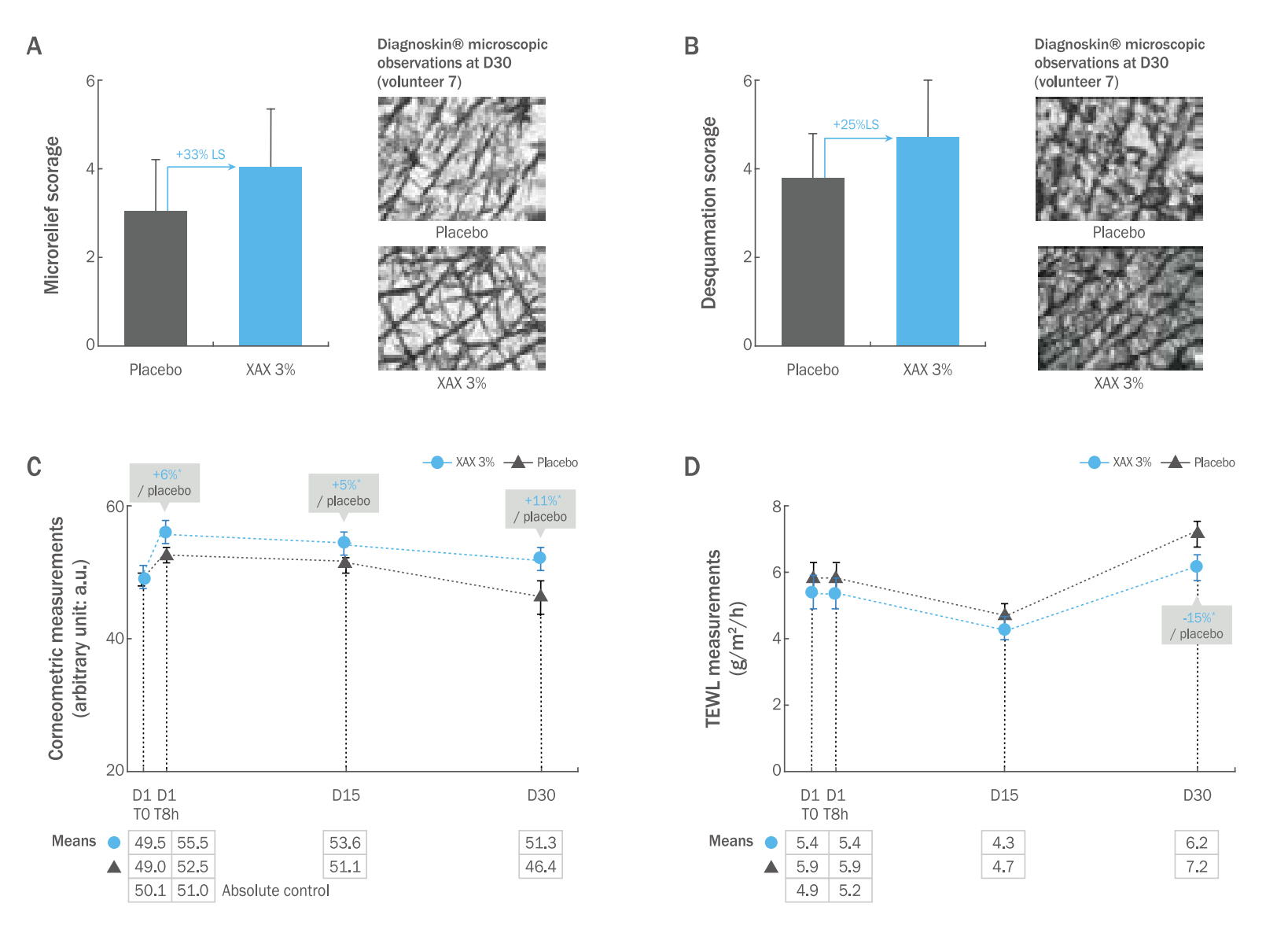
Figure 4.Clinical results on hands hydration (hydroalcoholic gels).(A) Results obtained after skin capacitance measures are expressed in arbitrary units (a.u.; y-axis), as well as the percentages of variation in comparison with T0; (B) Results on TEWL are expressed in g/m2/h (y-axis); Statistical analysis on comparaison of either glycerin or the combination glycerin+XAX with that of the placebo conditions; Student's t-test: *p<0.05, **p<0.01, ***p<0.001.
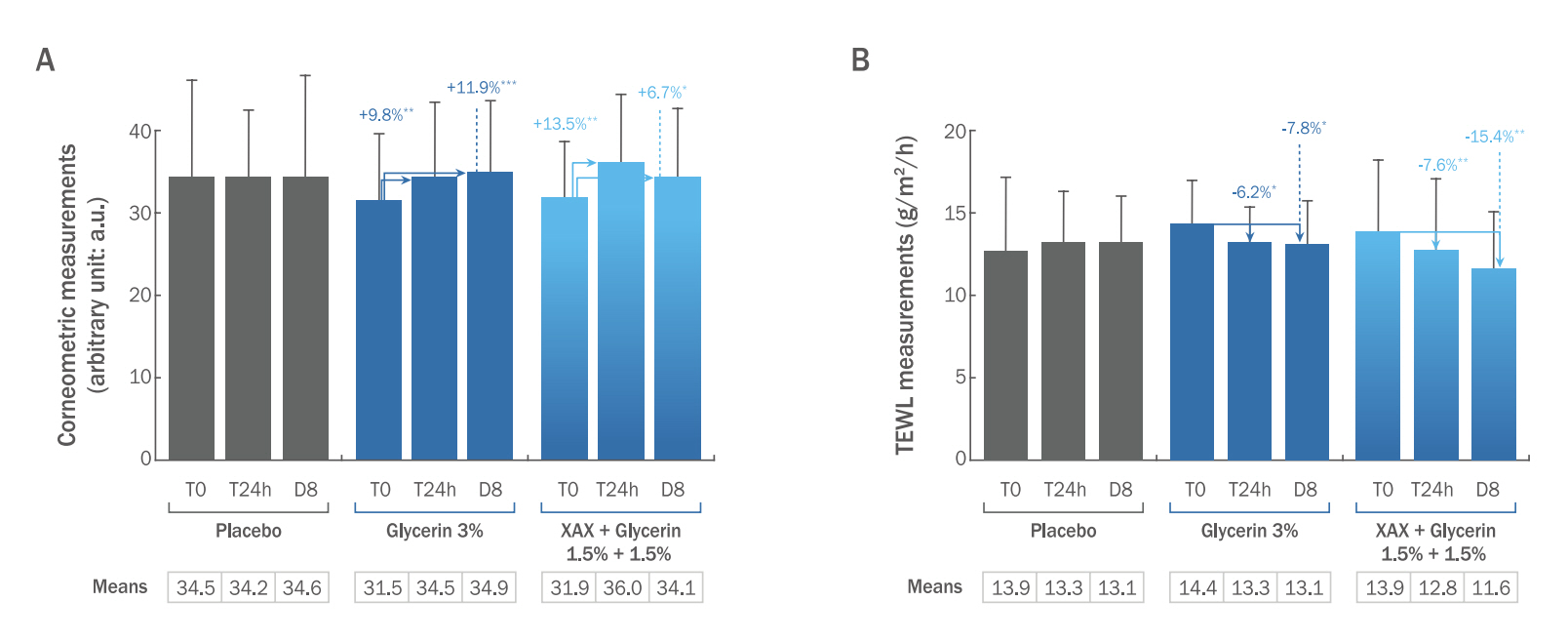
Figure 5.Schematic representation of XAX effects on skin moisturization-related biological targets (in vitro investigations).1- Reinforcement of barrier effect; 2- Optimization of water reserves; 3- Modulation of water circulation within epidermis.

Table 1.Compositions of formulations designed for topical applications Table 2.List of genes whose expression was upregulated by XAX-treatment in 3D-reconstructed epidermis ReferencesCrawford M, Dagnino L. Scaffolding proteins in the development and maintenance of the epidermal permeability barrier. Tissue Barriers 5: e1341969. 2017.
Draelos ZD. The science behind skin care: moisturizers. Journal of Cosmetic Dermatology 17: 138-144. 2018.
Faulkner R, Jo Y. Synthesis, function, and regulation of sterol and nonsterol isoprenoids. Frontiers in Molecular Biosciences 9: 1006822. 2022.
Goleva E, Berdyshev E, Leung DY. Epithelial barrier repair and prevention of allergy. Journal of Clinical Investigation 129: 1463-1474. 2019.
Guzman-Alonso M, Cortazar TM. Water content at different skin depths and the influence of moisturizing formulations. Household and Personal Care Today 11: 35-40. 2016.
Haftek M. Epidermal barrier disorders and corneodesmosome defects. Cell and Tissue Research 360: 483-490. 2015.
Henry J, Toulza E, Hsu CY, Pellerin L, Balica S, MazereeuwHautier J, Paul C, Serre G, Jonca N, Simon M. Update on the epidermal differentiation complex. Frontiers in Bioscience 17: 1517-1532. 2012.
Hogan MB, Peele K, Wilson NW. Skin barrier function and its importance at the start of the atopic march. Journal of Allergy 2012: 901940. 2012.
Jemec GBE, Na R. Hydration and plasticity following long-term use of a moisturizer: a single-blind study. Acta Dermato-Venereologica 82: 322-324. 2002.
Kuo IH, Carpenter-Mendini A, Yoshida T, McGirt LY, Ivanov AI, Barnes KC, Gallo RL, Borkowski AW, Yamasaki K, Leung Y. Activation of epidermal toll-like receptor 2 enhances tight junction function: implications for atopic dermatitis and skin barrier repair. Journal of Investigative Dermatology 133: 988-998. 2013.
Lefèvre-Utile A, Braun C, Haftek M, Aubin F. Five functional aspects of the epidermal barrier. International Journal of Molecular Sciences 22: 11676. 2021.
Mc Govern JA, Meinert C, de Veer SJ, Hollier BG, Parker TJ, Upton Z. Attenuated kallikrein-related peptidase activity disrupts desquamation and leads to stratum corneum thickening in human skin equivalent models. British Journal of Dermatology 176: 145-158. 2017.
Mojumdar E. H, Pham Q. D, Topgaard D, Sparr E. Skin hydration: interplay between molecular dynamics, structure and water uptake in the stratum corneum. Nature Scientific Reports 15712: 1-13. 2017.
Shi J, Barakat M, Chen D, Chen L. Bicellular tight junctions and wound healing. International Journal of Molecular Sciences 19: 3862. 2018.
Sun BK, Boxer LD, Ransohoff JD, Siprashvili Z, Qu K, LopezPajares V, Hollmig ST, Khavari PA. CALML5 is a ZNF750- and TINCR-induced protein that binds stratifin to regulate epidermal differentiation. Genes and Development 29: 2225-2230. 2015.
|
|
||||||||||||||||||||||||||||||||||||||