요약목적천연 화장품에 대한 수요가 증가함에 따라 적절한 생산 관리 및 품질 관리가 우선 되어야 한다. Chlorogenic aicd (CGA), epicatechin gallate (ECG) 및 rosmarinic acid (RMA)을 지표성분으로 선택하여 Ligularia stenocephala (LS; 곤달비), Orostachys japonica (OJ; 와송) 및 Lavandula angustifolia (LA; 라벤더) 50 % EtOH 추출물이 함유 된 화장품 조성물 LOL을 검증했다.
방법High-performance liquid chromatography with diode array (HPLC-DAD)를 사용하여 LOL (Ligularia stenofelphala, Orostachys japonica, Lavandula anfustifolia)의 세 가지 화합물을 동시에 분석했다. HPLC-DAD를 사용하여 선형성, 정확도 및 정밀도 분석을 수행했다. 그리고 DPPH 라디칼 소거 분석을 통해 항산화 효과를 평가했다.
결과세 가지 추출물 모두 DPPH 라디칼 소거 효과가 높았으며 검출 된 지표 성분은 유의 한 직선성을 나타냈다(R2≥0.9997). CGA, ECG 및 RMA의 검출 한계(LOD)는 각각 0.83 µg/mL, 0.33 µg/mL 및 0.20 µg/mL이며, CGA, ECG 및 RMA의 정량 한계(LOQ)는 각각 2.51 µg/mL, 0.99 µg/mL 및 0.61 µg/mL이다. 분석법 검증 결과, CGA, ECG 및 RMA의 허용 가능한 정밀도(일중 및 일간 정밀도)는 각각 1.77, 1.42, 0.80 % 및 1.09, 0.72, 1.42 %이고, 회수율은 각각 102.00 %-117.78 %, 94.40 %-108.06 % 및 93.79 %-107.05 %이다.
AbstractPurposeAs the demand for natural cosmetics increases, properly managing production and quality control should be prioritized. Chlorogenic acid (CGA), epicatechin gallate (ECG), and rosmarinic acid (RMA) were selected to validate the cosmetic composite LOL, a mixture of 50% EtOH extract of Ligularia stenocephala (LS), Orostachys japonica (OJ), and Lavandula angustifolia (LA).
MethodsHigh-performance liquid chromatography with a diode array detector (HPLC-DAD) was used to simultaneously analyze the three LOL compounds from LOL (Ligularia stenocephala, Orostachys japonica, Lavandula angustifolia). Linearity, accuracy, and precision analyses were performed using HPLC-DAD, and the anti-oxidative effects were evaluated through a 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical-scavenging assay.
ResultsAll three extracts showed a high DPPH radical-scavenging effect, and the detected marker compounds showed significant linearity (R2≥0.9997). The limit of detection (LOD) of CGA, ECG, and RMA was 0.83 µg/mL, 0.33 µg/mL, and 0.20 µg/mL, respectively. Meanwhile, the limit of quantification (LOQ) of CGA, ECG, and RMA was 2.51 µg/mL, 0.99 µg/mL, and 0.61 µg/mL, respectively. Method validation showed acceptable precision (intra-and inter-day precision, CGA, ECG, and RMA, 1.77, 1.42, 0.80% and 1.09, 0.72, 1.42%, respectively) and recovery (CGA, ECG, and RMA, 102.00%–117.78%, 94.40%-108.06%, and 93.79%–107.05%).
中文摘要目的 随着对天然化妆品需求的增加,应优先考虑妥善管理生产和质量控制。选择绿原酸(CGA)、表儿茶素没食子酸酯(ECG)和迷迭香酸(RMA)来验证化妆品复合物 LOL,这是一种由 50% 乙醇提取物的窄头胡瓜 (LS)、粳稻 (OJ) 和狭叶薰衣草的混合物(LA)。
方法 采用带二极管阵列检测器的高效液相色谱法(HPLC-DAD)同时分析来自 LOL(Liguaria stenocephala、Orostachys japonica、Lavandula angustifolia)的三种 LOL 化合物。使用 HPLC-DAD 进行线性、准确度和精密度分析,并通过 DPPH 自由基清除试验评估抗氧化作用。
结果 三种提取物均显示出较高的 DPPH 自由基清除作用,且检测到的标记化合物呈显着线性(R2≥0.9997)。CGA、ECG 和 RMA 的检测限 (LOD) 分别为 0.83 µg/mL、0.33 µg/mL 和 0.20 µg/mL。同时,CGA、ECG 和 RMA 的定量限 (LOQ) 分别为 2.51 µg/mL、0.99 µg/mL 和 0.61 µg/mL。方法验证显示,CGA、ECG 和 RMA的可接受的精密度(日内和日间精密度)分别为 1.77、1.42、0.80% 和 1.09、0.72、1.42%; CGA、ECG 和 RMA的回收率分别为102.00%– 117.78%、94.40%-108.06% 和 93.79%–107.05%。
IntroductionRecently, developments in biotechnology have brought about an increase in the demand for cosmetics manufactured from natural ingredients, including herbal medicine. Skin whitening cosmetics use organic materials, such as extracts of Vaccinium vitis-idaea or Broussonetia kazinoki, after hydroquinone was found to have carcinogenic properties. The demand for multifunctional cosmetics is on the rise, and complex compositions of two or more organic materials have also been popularized (Chanchal & Swarnlata, 2008). However, relevant technical needs and, quality control standards in manufacturing are insufficient. Without setting a benchmark for quality, various bodily mechanisms cannot be considered, and component destruction occurs during the distribution process based on raw material stability. A mixture of two incompatible ingredients may result in an antagonistic effect and without a proper understanding of these ingredients, products can become harmful to consumers (Espinosa-Leal & Garcia-Lara, 2019; Mellou et al., 2019; Nohynek et al., 2010).
In Korea, standards and methods for testing the functional or active ingredients of functional food and herbal medicines have been legislated, but standardization is not yet required for natural cosmetics. If the natural extracts' raw materials cannot be disclosed accurate, the product' s reliability can be questioned. Therefore, a product' s quality must be assured to improve customer satisfaction (Chang, 2003).
Numerous analytical techniques have been used to quantify cosmetic ingredients through high-performance liquid chromatography (HPLC), such as HPLC coupled with other detectors, including ultraviolet (UV) detectors, mass spectrometers, and diode array detectors (DADs) (Papageorgiou et al., 2020). However, high-performance liquid chromatography with a diode array detector (HPLC-DAD) is highly recommended for analyzing herbal combinations because it offers 3D analyses and a wide array of mobile phases while being more affordable and resistant to contamination. This method is beneficial for simultaneously analyzing samples with mixed components with of different absorption wavelengths (Czerwińska et al., 2020; Elansary et al., 2019; Ham et al., 2018; Xiao et al., 2017).
This study attempted to develop a combination of natural cosmetic materials according to the recent increase in demand for natural cosmetics. A composite of LOL was prepared by securing 50% ethanol extracts of three medicinal herbs cultivated in Namwon city; Lavender (Lavandula anfustifolia) native to the west, narrow-head ragwort (Ligularia stenofelph), and rock pine (Orostachys japonica) native to the east. Each component's DPPH scavenging ability was measured, and a simultaneous analysis method of the index component of the combination LOL using HPLC-DAD was developed.
A Ligularia stenocephala (Maxim.) Matsum. & Koiz (LS), Orostachys japonica (Maxim.) A. Berger (OJ), and Lavandula angustifolia Mill (LA) (LOL) composite contains 50% EtOH extract of LS narrow-head ragwort, OJ rock pine, and LA lavender. According to the International Nomenclature of Cosmetic Ingredients (INCI), LS and OJ are named Ligularia stenocephala extract and Orostachys japonica extract, respectively.
The LS perennial herb, which belongs to the Asteraceae family, can be found in Korea (Jeollanam-do), Japan, Taiwan, and China. Young leaves are harvested and eaten as herbs, and in oriental medicine, the root is used as medicine for bowel disorders and gynecological diseases (Kim et al., 2012b). This herb has numerous benefits, including producing antioxidants, reducing melanin production, lessening wrinkles while also possessing hepatoprotective, anti-diabetic, and anti-ulcerogenic effects (Lee et al., 2010; Roh et al., 2009). Several phytochemicals identified from LS include N-phenyl-2-naphthylamine, neophytadiene, vanillin, triterpenoid derivatives, 4-hydroxy-acetophenone, chlorogenic acid (CGA), and caffeic acid, ligulacephalins A, B, C, and euparin (Todoya et al., 2005).
The OJ of a perennial flowering plant in the Crassulaceae family, commonly referred to as a rock pine, is found on mountain rocks in Korea and Japan. The rock pine possesses anti-cancer properties (Kim et al., 2012a), enhances hepatic alcohol dehydrogenase (Hur & Park, 2006), protects neuronal cells from apoptosis (Yoon et al., 2000), and demonstrates antioxidative (Choi et al., 2008) and anti-inflammatory, and inhibitory effects on collagenase, elastase, and tyrosinase (Im et al., 2017). Several phytochemicals identified in OJ include epicatechin, epicatechin gallate (ECG), gallic acid, kaempferol, kaempferol 3-O-α-l-rhamnopyranoside, quercetin, quercetin 3-O-β-D-glucopyranoside, and pyrogallol (Kim et al., 2008).
The LA belongs to the Lamiaceae family, and lavender essential oil has been used for treating neurological disorders and rheumatism while demonstrating antibacterial effects (Cavanagh & Wilkinson, 2002; Ha et al., 2019). Lavender extract mixed in hot water and ethanol also contains antioxidants and display whitening and sebum inhibitory effects (Carrasco et al., 2016; Hus et al., 2007). The reported substances in LS include rosmarinic acid (RMA), luteolin, apigenin, apigenin 7-O-β-Dglucoside, luteolin 7-O-β-D-glucuronide, linalool, linalyl acetate, (E)-β-caryophyllene, eucalyptol, and camphor (Zhao et al., 2015; Andrys et al., 2018).
This study aims to develop a new method that simultaneously analyzes each marker compound from the LOL composite using HPLC-DAD. Several validation methods were conducted, including tests for accuracy, specificity, precision, and the limit of detection (LOD), the limit of quantitation (LOQ), range, and linearity.
Methods1. Preparation of materialsThe samples of LS, OJ, and LA used in the composite were harvested and purchased in Namwon City (Korea). After drying at 40℃ for 72 h with a dryer (LD9013; L'EQUIP Co., Korea), each herb was pulverized with a blender (HMF-3600TG; Hanil Electric, Korea). A total of 2 L of 50% ethanol per 100 g was used for extraction at 80℃ for 4 h, and the extract was filtered twice with nonwoven fabric and filter paper. The filtered extract used as a sample first was concentrated at 50℃ with a vacuum concentrator (R-100; BUCHI, Switzerland), then freeze-dried with a freeze dryer (MCFD8508; Ilshin Biobase Co., Korea), which resulting in a 17% extraction yield. The three herbs used in the experiment were authenticated by Professor Dae Keun Kim of the Woosuk University's Department of Pharmacy.
2. ReagentsStandard CGA, ECG, and RMA (Biopurify Phytochemicals, China) with more than 98% purity were utilized for this study. In addition, the methanol and acetonitrile (J.T. Baker, Phillipsburg, USA) used in this study are HPLC-grade organic solvents, and the acid used was formic acid (Fluka, Germany).
3. DPPH radical scavenging assayThe DPPH radical scavenging activity was measured by using a modified Blois method (Blois, 1958). After adding 247.5 μL of a 0.2 mM DPPH (Sigma-Aldrich, USA) solution to 2.5 μL of the sample, a reaction occurs in the dark at room temperature for 20 min. Measurements were taken at 517 nm using an ELISA reader (Bio-Rad, USA) after the reaction occurs. The sample's concentration when the absorbance of DPPH decreased by 50% (RC50) was determined, and each sample was tested three times. L-ascorbic acid (Sigma-Aldrich) was the positive control (Ham et al., 2018).
4. Pretreatment of samplesFor HPLC analysis, 20 mg of an equal composite of the three extracts was taken and ultrasonically extracted with 1 mL of 70% methanol for 30 min before being filtered through a 0.2 µm membrane filter (PALL Co., USA). The marker component of the standard product was also obtained similarly.
5. Instrumentation and Chromatographic conditionsFor the marker components' qualitative and quantitative analyses, a Hitachi Chromaster HPLC CM5000 system (Hitachi, Japan) was used, and detection was performed at multiple wavelengths using a diode array detector (DAD). YMC-Pack ODS-AM, C18 column (250×4.6 mm i.d; YMC, Japan) was used at 40℃. Its mobile phase was composed of water (A) containing 0.1% formic acid and acetonitrile (B) containing 0.1% formic acid with a flow rate of 1.0 mL/min. The slope of the mobile phase was 0-10 min, (A): 100%, 10-55 min, (A): 60%, 55-60 min, (A): 0%, 60-65 min, (A): 0%, 65-70 min, (A): 100%. Detection wavelengths were set at 213 nm, 326 nm, and 329 nm, and HPLC data analysis was performed using Agilent Open Lab Software (Agilent, CA).
6. Methodology validationThe methodology was veridated by determining the linearity, range, LOD, LOQ, accuracy, precision, and content evaluation based on the Guidelines for Validation of Pharmaceutical Drugs from the Ministry of Food and Drug Safety (NIFDS, 2015).
7. Linearity and rangeLinearity was evaluated by preparing a calibration curve and calculating a regression equation based on the results obtained after diluting the marker compounds a three different concentrations (1, 50, and 100 µg/mL).
8. SpecificityAfter preparing a composite of the three plant extracts and a standard mixture of each indicator component, the chromatogram was visually evaluated. The ultraviolet (UV) spectrum of the corresponding indicator component was assessed by extracting the standard mixture's chromatogram for comparison.
9. Accuracy and precisionConsequently, both accuracy and precision were measured by comparing the integral values of the marker compounds' peaks. The accuracy was evaluated by adding three different concentrations of the standard product to the sample before measuring the recovery rate. In comparison, precision was determined using relative standard deviation (RSD) through intraday and interday tests. Each experiment was evaluated by calculating the results' RSDs obtained through three repeated experiments with three concentrations of mixed standard solutions.
10. Limit of detection (LOD) and limit of quantification (LOQ)The LOD and LOQ were calculated using the following formula (Chanchal & Swarnlata, 2008) based on the prepared calibration curve, where σ is the standard deviation of the response and S is the slope of the calibration curve.
LOD=3.3 (σ/S)
LOQ=10 (σ/S)
Result and Discussion1. DPPH radical-scavenging activity
Table 1 shows the sample's concentration required for 50% DPPH radical scavenging, which identifies the measured DPPH scavenging activities of the EtOH extract of LS, OJ, LA, and the composite. The OJ showed the highest DPPH scavenging activity of 48.21±4.0 µg/mL and its combination with LA showed RC50 at 58.06±1.3 µg/mL. Meanwhile, the LOL composite showed RC50 at 67.73±2.6 µg/mL, and the positive control L-ascorbic acid showed RC50 at 19.81 µg/mL. Choi et al. (2008) reported that the MeOH extract of OJ could donate electrons, act as a reducing agent, and demonstrate the superoxide dismutase-like activity, suggesting that harvesting around August to October contributed to high anti-oxidative activityies. Im et al. (2017) reported that EtOH extracts of OJ inhibited the enzyme activities of collagenase, elastase, and tyrosinase by 85%, 45%, and 70%, respectively, at a concentration of 500 µg/mL. Meanwhile, Kim et al. (2012a) reported that the ethyl acetate fraction of LS had RC50 values of 280 µg/mL for DPPH radical scavenging, suggesting that LS can act as a preservative for food, medicine, or cosmetics because it had excellent antibacterial properties against Bacillus cereus. Meanwhile, LA is used as a cosmetic ingredient because it possesses anti-aging, antioxidative, and inhibitory properties on tyrosinase activity (Hsu et al., 2007). The three plants' phenolic compounds had a high radical scavenging activity (Kadoma & Fujisawa, 2008), and the composite, as a cosmetic formulation, could prevent the overproduction of melanin through its anti-oxidative properties.
2. Qualitative and quantitative analysis of marker compounds.The study was conducted to predict several components to determine the marker compounds of EtOH extracts of LS, OJ, and LA. CGA, ECG, and RMA were selected, which were detected with higher content from the standard calibration curves obtained through liquid chromatography/mass spectrometry (LC/MS) analysis (Ham et al., 2018). The structures of these compounds are shown in Figure 1. Three marker compounds were analyzed with water containing 0.1% formic acid and acetonitrile made up of 0.1% formic acid through HPLC-DAD.
3. Establishment of simultaneous analysis conditionsThe marker compounds demonstrated showed high absorbance at 213 nm, 326 nm, and 329 nm on the HPLC-DAD analysis's UV absorbance spectrum (Figure 3). In Figure 2A, a mixture of the standards' chromatogram showed that the CGA, ECG, and RMA peaks showed different retention times (27.78 min, 34.50 min, and 39.23 min, respectively) without interference. These results demonstrated that the conditions of simultaneous analysis of three marker compounds were established.
4. SpecificityAs shown in Figure 2B, the LOL composite's chromatogram showed that the retention times of the three marker components' peaks from LOL were identical to the retention times of the standard materials. This result determined that CGA, ECG, and RMA were present in the LOL composite because its peaks on the UV absorbance spectrum are identical to the UV spectrum of standard products (Figure 3). Meanwhile, the chromatograms at 213 nm showed an increase in peak intensity with increasing absorbance of ECG, and the chromatograms at 326 nm and 329 nm revealed an increase in peak intensity with increasing CGA and RMA absorbance. The results confirmed that each marker component's peak increased on the LOL chromatogram when each standard material was injected with LOL as an internal standard (data not shown). Figure 2B also shows several peaks beside the marker components.
5. Linearity, LOD and LOQLinear regression analysis of each marker compound was evaluated using R2, range, LOD, and LOQ (Table 2). All three marker compounds exhibited linearity with a correlation coefficient of R2>0.9997 in a 1-100 µg/mL concentration range. The LOD of CGA, ECG, and RMA were determined to be 0.83 µg/mL, 0.33 µg/mL, and 0.20 µg/mL, respectively, and the LOQ range of CGA, ECG, and RMA were 2.51 µg/mL, 0.99 µg/mL, and 0.61 µg/mL, respectively.
6. RecoveryAs shown in Table 3, the recovery test results measured after adding three concentrations (low, medium, and high) of each marker standard to the extracts, showed that the recovery of CGA was 102.00%-117.78%, and the relative standard deviation ranged 0.21%-1.38%. The ECG recovery rate was 94.40%-108.06%, and its relative standard deviation was around 0.84%-1.95%. Meanwhile, RMA showed a recovery rate between 93.79%-107.05% and a relative standard deviation of 0.66%-1.41%. The recovery data of all three compounds were between 93.79% and 117.78% and had RSD values less than 1.95%, demonstrating this method's reliability under established conditions.
7. Accuracy and precisionThe method's accuracy and precision were evaluated using triplicate analysis once a day over three days, and intraday accuracy of CGA, ECG, and RMA was 107.06%, 102.25%, and 92.24%, respectively. Conversely, inter-day accuracy was 110.39%, 104.03%, and 89.94% for CGA, ECG, and RMA, respectively. The recovery rates of all three marker compounds were between 89.94% and 110.39%, which are within 20% of the reference values of the Ministry of Food and Drug Safety's guidelines. All three markers had a range of 0.80%-1.77% for intraday RSD, and their inter-day RSD had a range of 0.72%-1.42%. These results reflect the guidelines' RSD of less than 5% (Table 4). Table 5 outlines the results of the quantitative analysis of the LOL composite's marker components, where CGA content was 13.99 µg/mg (1.4%), ECG was 8.28 µg/mg (0.8%), and RMA was 8.40 µg/mg (0.8%) with 0.76%-1.54% RSD. This validation method can control the quality of the cosmetic ingredients, such as LS, OJ, and LA, registered on INCI.
ConclusionTo develop a complex composition of natural cosmetic materials, 50% EtOH extracts of LS, OJ, and LA-which have relatively high DPPH radical scavenging abilities-were selected, and CGA, ECG, and RMA were identified as marker compounds. The HPLC-DAD method was used to analyze the marker compounds in the LOL composite, and validation through linearity, accuracy, and precision measurements was established. The contents of the three marker compounds were 13.99 µg/mg CGA, 8.28 µg/mg ECG, and 8.40 µg/mg RMA, and the results of the analysis could be used to improve quality control for natural cosmetics. Further analysis of other physiological activities is necessary to develop the LOL composite as a superior natural cosmetic composition.
AcknowledgementsThis work was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through the Agriculture, Forestry and Livestock Food Research and Development project, funded by the Ministry of Agriculture, Food and Rural Affairs (MAFRA) (Grant number: 317024054SB020). The authors would also like to thank the Namwon City Cosmetic Industry Support Center for supplying the extract used in the study.
NOTESAuthor's contribution
JEK, ACS, JYJ and JYL contributed equally to this work. JYL supervised the project, and JEK, ACS, JYJ performed experimental design and analysis for HPLC analysis with the help of JYL. JYL and JEK wrote the manuscript together.
Author details
Ju Eun Kim (Ph.D), Department of Pharmacy, Woosuk University, 443, Samnye-ro, Samnye-eup, Jeollabuk-do 55338, Korea; Abinash Chandra Shrestha (MS), Department of Pharmacy, Woosuk University, 443, Samnye-ro, Samnye-eup, Jeollabuk-do 55338, Youn Jeong Jo (Gradutre student), Department of Pharmacy, Woosuk University, 443, Samnye-ro, Samnye-eup, Jeollabuk-do 55338, Jae Yoou Leem (Professor), Department of Pharmacy, Woosuk University, 443, Samnye-ro, Samnye-eup, Jeollabuk-do 55338, Korea.
Figure 2.HPLC profile in the simultaneous analysis of the LOL(A) Representative chromatogram of standards mixture solution at 213 nm, 326 nm, and 329 nm, respectively. (B) Representative chromatogram of LOL, cosmetic composite at 213 nm, 326 nm, and 329 nm, respectively. CGA, chlorogenic acid; ECG, epicatechin gallate; RMA, rosmarinic acid; LOL, mixture of Ligularia stenocelphala, Orostachys japonica and Lavandula angustifolia.
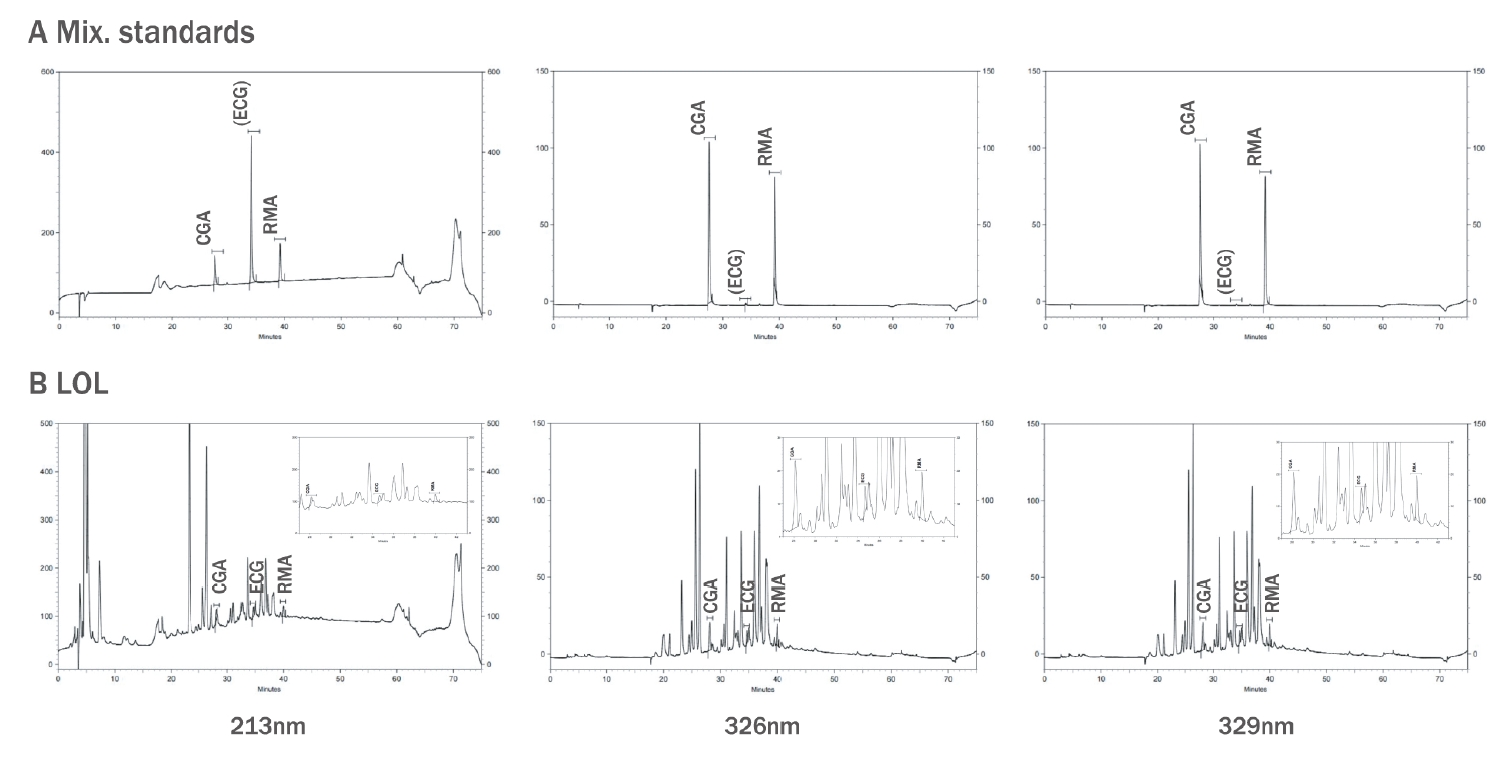
Figure 3.UV spectra of standard marker compound and marker compound in LOL(A) UV Spectra of three marker compound from the standard mixture and (B) from the mixture of three herbs, LOL (Ligularia stenocelphala, Orostachys japonica, and Lavandula angustifolia).
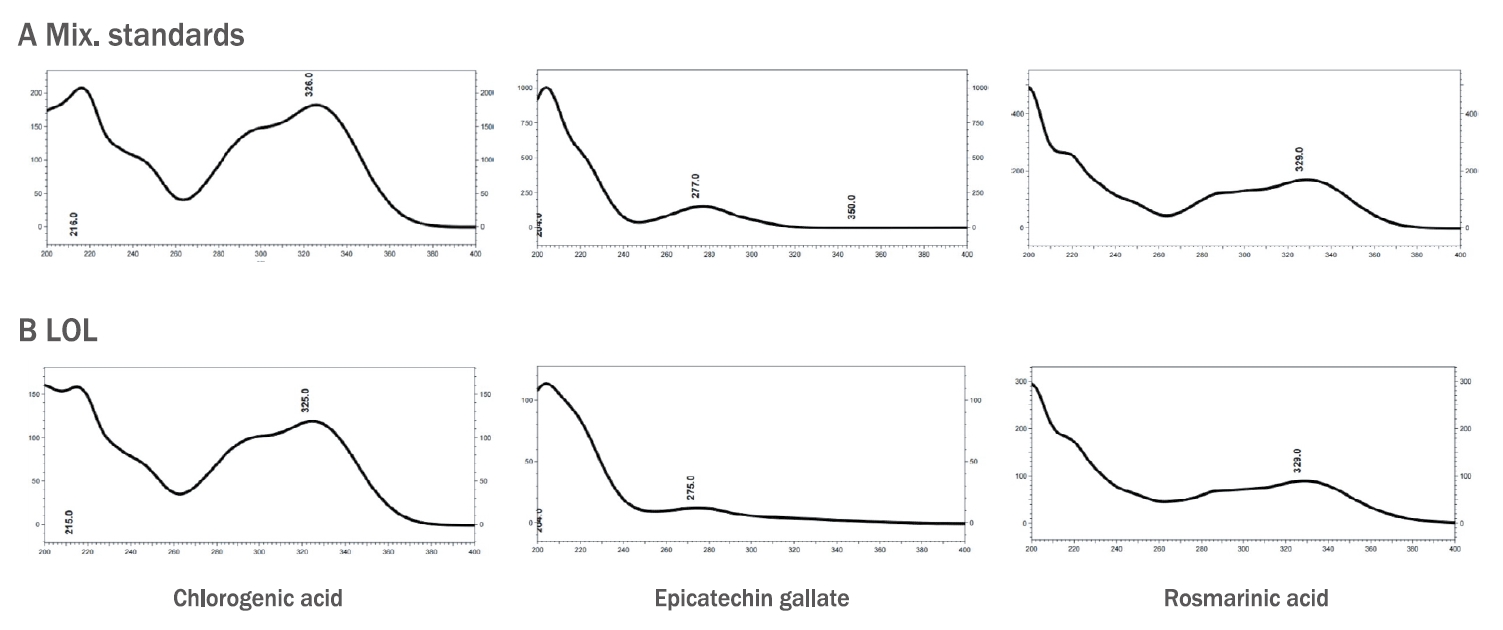
Table 1.Concentration (RC50) value of 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging activity
Table 2.HPLC calibration data for CGA, ECG, and RMA Table 3.Recovery levels of CGA, ECG, and RMA Table 4.Accuracy and precision data for the quantitative determination of CGA, ECG, and RMA
ReferencesAndrys D, Adaszyńska-Skwirzyńska M, Kulpa D. Essential oil obtained from micropropagated lavender, its effect on HSF cells and application in cosmetic emulsion as a natural protective substance. Natural Product Research 32: 849-853. 2018.
Blois MS. Antioxidant determinations by the use of a stable free radical. Nature 181: 1199-1200. 1958.
Carrasco A, Martinez-Gutierrez R, Tomas V, Tudela J. Lavandula angustifolia and Lavandula latifolia essential oils from Spain: aromatic profile and bioactivities. Planta Medica 82: 163-170. 2016.
Cavanagh HMA, Wilkinson JM. Biological activities of lavender essential oil. Phytotherapy Research 16: 301-308. 2002.
Chanchal D, Swarnlata S. Novel approaches in herbal cosmetics. Journal of Cosmetic Dermatology 7: 89-95. 2008.
Chang IS. Present and future of functional cosmetics. Journal of the Society of Cosmetic Scientists 29: 149-178. 2003.
Choi SY, Chung MJ, Sung NJ. Studies on the antioxidative ability of methanol and water extracts from Orostachys japonicus A. Berger according to harvest times. The Korean Journal of Food and Nutrition 21: 157-164. 2008.
Czerwińska ME, Kalinowska E, Popowski D, Bazylko A. Lamalbid, chlorogenic acid, and verbascoside as tools for standardization of Lamium album flowers-development and validation of HPLC-DAD method. Molecules 25: 1721. 2020.
Espinosa-Leal CA, Garcia-Lara S. Current methods for the discovery of new active ingredients from natural products for cosmeceutical applications. Planta Medica 85: 535-551. 2019.
Elansary HO, Szopa A, Klimek-Szczykutowicz M, Jafernik K, Ekiert H, Mahmoud EA, Abdelmoneim Barakat A, El-Ansary DO. Mammillaria species-polyphenols studies and anti-cancer, anti-oxidant, and anti-bacterial activities. Molecules 25: 131. 2019.
Ha JH, Kim AR, Lee KS, Xuan SH, Kang HC, Lee DH, Cha MY, Kim HJ, An M, Park SN. Anti-aging activity of Lavandula angustifolia extract fermented with Pediococcus pentosaceus DK1 isolated from Diospyros kaki fruit in UVB-irradiated human skin fibroblasts and analysis of principal components. Journal of Microbiology and Biotechnology 29: 21-29. 2019.
Ham HN, Shrestha AC, Kim JE, Lee TB, Yoo BW, Kim MS, Kim KS, Cha JS, Lee YM, Kim JY, et al. Simultaneous analysis of the compounds of natural cosmetic resources containing Chrysanthemum zawadskii, Perilla frutescens, Rosa multiflora and their anti-oxidative activity. Korean Journal of Pharmacognosy 49: 312-321. 2018.
Hsu CK, Chang CT, Chung YC. Inhibitory effects of the water extracts of Lavendula sp. on mushroom tyrosinase activity. Food Chemistry 105: 1099-1105. 2007.
Hur JM, Park JC. Effects of the aerial parts of Orostachys japonicus and its bioactive component on hepatic alcohol-metabolizing enzyme system. Journal of Medicinal Food 9: 336-341. 2006.
Im DS, Lee JM, Lee J, Shin HJ, No KT, Park SH, Kim K. Inhibition of collagenase and melanogenesis by ethanol extracts of Orostachys japonicus A. Berger: possible involvement of Erk and Akt signaling pathways in melanoma cells. Acta Biochimica et Biophysica Sinica (Shanghai) 49: 945-953. 2017.
Kadoma Y, Fujisawa S. A comparative study of the radicalscavenging activity of the phenolcarboxylic acids caffeic acid, p-coumaric acid, chlorogenic acid and ferulic acid, with or without 2-mercaptoethanol, a thiol, using the induction period method. Molecules 13: 2488-2499. 2008.
Kim HJ, Lee JY, Kim SM, Park DA, Jin C, Hong SP, Lee YS. A new epicatechin gallate and calpain inhibitory activity from Orostachys japonicus. Fitoterapia 80: 73-76. 2008.
Kim JY, Jung EJ, Won YS, Lee JH, Shin DY, Seo KI. Cultivated Orostachys japonicus induces apoptosis in human colon cancer cells. Korean Journal of Food Science and Technology 44: 317-323. 2012a.
Kim KH, Kim NY, Kim SH, Han IA, Yook HS. Study on antioxidant effects of fractional extracts from Ligularia stenocephala leaves. Journal of the Korean Society of Food Science and Nutrition 41: 1220-1225. 2012b.
Lee BI, Agung N, Moch SB, Choi J, Lee KR, Kim WB, Lee KT, Lee JD, Park HJ. Anti-ulcerogenic effect and HPLC analysis of the caffeoylquinic acid-rich extract from Ligularia stenocephala. Biological & Pharmaceutical Bulletin 33: 493-497. 2010.
Mellou F, Varvaresou A, Papageorgiou S. Renewable sources: applications in personal care formulations. International Journal of Cosmetic Science 41: 517-525. 2019.
NIFDS. Drug validation guidelines. National Institute of Food and Drug Safety Evaluation. Seoul. pp1-23. 2015.
Nohynek GJ, Antignac E, Re T, Toutain H. Safety assessment of personal care products/cosmetics and their ingredients. Toxicology and Applied Pharmacology 243: 239-259. 2010.
Papageorgiou S, Varvaresou A, Panderi I, Giannakou M, Spiliopolou C, Athanaselis S. Development and validation of a reversed-phase high-performance liquid chromatographic method for the quantitation and stability of α-lipoic acid in cosmetic creams. International Journal of Cosmetic Science 42: 221-228. 2020.
Roh EJ, Kim YS, Kim BG. Effect of antioxidation and inhibition of melanogenesis from Ligularia stenocephala extract. Journal of the Korean Applied Science and Technology 26: 87-92. 2009.
Todoya K, Yaoita Y, Kikuchi M. Three new dimeric benzofuran derivatives from the roots of Ligularia stenocephala MATSUM. et KOIDZ. Chemical & Pharmaceutical Bulletin 53: 1555-1558. 2005.
Xiao W, Peng Y, Tan Z, Lv Q, Chan CO, Yang J, Chen S. Comparative evaluation of chemical profiles of pyrrosiae folium originating from three pyrrosia species by HPL-CDAD combined with multivariate statistical analysis. Molecules 22: 2122. 2017.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||