요약목적본 연구는 유자 정유의 피부 항노화 특성을 평가하고, 생물학적 활성을 입증하는 것을 목표로 한다. 이를 위해 체외 실험과 임상 시험을 통해 유자 정유의 잠재력을 확인하고자 한다.
방법유자 정유의 화학적 성분을 가스 크로마토그래피-질량 분석법(GCMS)을 이용하여 분석하였다. 인체 표피 각질 세포(HEKs)와 인체 피부 섬유아세포 (HDF)에서 유자 정유의 세포 생존율, NO 생성 억제, 항염증 활성, 콜라겐 합성 및 콜라겐 분해에 미치는 영향을 평가하였다. 또한 피부 자극 가능성을 평가하기 위해 임상 참가자에게 피부 패치 테스트를 실시하였으며, 20명의 여성 참가자를 대상으로 피부 탄력에 미치는 유자 정유의 영향을 임상 평가하였다.
결과유자 정유는 효과적으로 NO 생성을 억제하였으며, TNF-α, IL-1β, IL-6 및 NF-κB를 포함한 염증 표지자의 발현을 유의하게 억제하였다. 그뿐만 아니라 유자 정유는 콜라겐 분해 인자인 MMP-1와 MMP-3 발현을 유의하게 억제하였으며, 콜라겐 합성과 관련된 COL1A1 및 COL3A1 유전자의 발현을 농도 의존적으로 증가시켰다. 20명의 여성 참가자에서는 유자 정유 에멀젼 처리 그룹에서 피부 탄력이 유의하게 개선되었으며, 피부 패치 테스트 결과 유자 정유 에멀젼이 피부 자극, 민감성피부자극, 누적자극 및 감작성 평가 시 안전성이 확인되었다.
AbstractPurposeThis study assessed Citrus junos essential oil (CJEO) as an anti-aging agent for improving skin health.
MethodsCJEO was extracted from authenticated C. junos fruit peels, and its chemical composition was analyzed using a gas chromatographymass spectrometry (GC-MS). In vitro experiments were conducted on human epidermal keratinocytes (HEKs) and human dermal fibroblasts (HDFs) to evaluate CJEO’s impact on cell viability, anti-inflammatory activity, collagen synthesis, and collagen degradation. The expression of TNF-α, IL-1β, IL-6, NF-κB, MMP-1, MMP-3, COL1A1, and COL3A1 was quantified using RT-qPCR. A dermal patch test was performed on human participants to assess potential skin irritation, and clinical evaluations were conducted on 20 female participants to analyze CJEO’s effects on skin elasticity.
ResultsCJEO effectively inhibited nitric oxide production and significantly suppressed the expression of inflammatory markers, including TNF-α, IL-1β, IL-6, and NF-κB, as well as collagen degradation markers MMP-1 and MMP-3. Furthermore, CJEO treatment upregulated the expression of genes associated with collagen synthesis, specifically COL1A1 and COL3A1. Notably, significant improvements in skin elasticity were observed in the 20 female participants. The dermal patch test indicated excellent tolerance of the CJEO emulsion without causing irritation.
ConclusionsCJEO exhibits promising potential as a bioactive ingredient in anti-aging cosmeceuticals. Its anti-inflammatory properties, ability to stimulate collagen synthesis, suggest efficacy in reducing skin aging and promoting overall skin health. Additionally, the dermal patch test confirms CJEO’s safety, supporting its suitability for use in cosmetic formulations. These findings endorse CJEO as an effective and safe component in anti-aging skincare products.
中文摘要方法 从正宗的香橙果皮中提取CJEO,采用气相色谱-质谱法(GC-MS)分析其化学成分。对人表皮角质形成细胞 (HEK) 和人真皮成纤维细胞 (HDF)进行体外实验,以评估CJEO对细胞活力、抗炎活性、胶原蛋白合成和胶原蛋白降解的影响。使用RT-qPCR定量分析TNF-α、IL-1β、IL-6、NF-κB、MMP-1、MMP-3、COL1A1和COL3A1的表达。对受试者进行了皮肤斑贴测试,以评估潜在的皮肤刺激,并对20名女性受试者进行了临床评估,以分析CJEO对皮肤弹性的影响。
IntroductionSkin aging is a multifaceted process influenced by intrinsic and extrinsic factors, including genetics and environmental exposure (Papakonstantinou et al., 2012). It leads to the development of wrinkles, loss of elasticity, and thinning of the skin, affecting a person's social life (Longo, 2016). The cosmetics industry is continuously explores active ingredients to prevent and reduce skin aging, with cosmeceuticals playing a vital role in anti-aging skin care (Martin & Glaser, 2011). Besides addressing the signs of aging, cosmeceutical products should also promote "healthy aging" by providing psychological tranquility and alleviating stress (Ganceviciene et al., 2012). Chronic psychological stress accelerates skin aging by disrupting the skin barrier, activating the hypothalamic-pituitary-adrenal axis, and increasing reactive oxygen species (ROS) production (Dunn & Koo, 2013).
Moreover, the aging process is linked to the activation of inflammatory pathways in the skin, which is commonly referred to as "inflammaging" (Neves & Sousa-Victor, 2020). UVB radiation plays a significant role in skin damage and aging by stimulating cytokines and increasing the production of ROS (Abbas et al., 2018; Lago & Puzzi, 2019). Key cytokines such as IL-1β, IL-6, and TNF-α known to induce chronic inflammation and initiate the breakdown of collagen by upregulating MMP (Borg et al., 2013). Another major player in cellular inflammation, NF-κB, is upregulated by UV irradiation, resulting in chronic inflammation and photogenic reactions (Ansary et al., 2021; Pittayapruek et al., 2016). TNF-α, which plays a crucial role in skin inflammation, can cause irreversible damage to the epidermis (Bashir et al., 2009; Mirastschijski et al., 2019). Excessive production of IL-6, TNF-α, and IL-1 has been associated with skin damage and an increased risk of melanoma (Tam & Stepień, 2011).
Skin aging is a result of gradual depletion of collagen and elastin in the dermal connective tissue, leading to thinner skin with reduced mechanical strength and increase in the formation of wrinkles (Parente et al., 2015; Varani et al., 2001). The degradation of collagen and elastin primarily occurs due to the activity of matrix metalloproteinases (MMPs), which are zinc-containing proteinases capable of breaking down various extracellular matrix proteins (Nelson et al., 2000; Wang et al., 2008). Exposure to UV radiation can significantly increase levels of multiple MMPs, including MMP-1, MMP-3, and MMP-9, which are highly regulated by AP-1 (Quan et al., 2009). These MMPs possess the ability to break down collagen fibers, thereby contributing to the formation of wrinkles and tissue damage during the aging process of the skin (Pittayapruek et al., 2016; Shen et al., 2019).
Botanical ingredients and their derived products are widely utilized as major cosmeceutical agents in cosmetics due to the increasing awareness of health benefits and the preference for environmentally friendly consumer behavior (Amberg & Fogarassy, 2019; Kang et al., 2021; Sharmeen et al., 2021). Essential oils, which are complex mixtures of lipophilic plant metabolites with a molecular weight below 300 atomic mass units, exhibit beneficial bioactive properties owing to their low molecular weight and lipophilicity (Arena et al., 2021; Kazemi et al., 2020; Modarresi et al., 2019). These oils are extracted from various plant parts, such as leaves, flowers, barks, rhizomes, roots, and citrus fruit peels (Miguel, 2010; Je et al., 2021), and find applications in aromatherapy, pharmaceuticals, food, and clinical medicine industries (Firenzuoli et al., 2014). Inhaling or topically applying essential oils can restore mental and physical balance, alleviate stress, and provide skin care benefits (Aburjai & Natsheh, 2003; Ali et al., 2015; Jung & Lee, 2022). Additionally, essential oils serve as fragrances, preservatives, and active agents in cosmetics, apart from their pleasant aroma (Burnett et al., 2019; Sharmeen et al., 2021). Citrus essential oils, extracted from citrus fruit peels are classified into sweet and sour varieties, with the sour citrus being preferred in cosmetics due to its refreshing scent (Fukumoto et al., 2008; Nguyen & Sawamura, 2008). The volatile nature of essential oils enables them to interact with receptors in the central nervous system, influencing memory and mood through inhalation (Buchbauer & Jirovetz, 1994; Hongratanaworakit, 2004).
Citrus junos Siebold ex Tanaka (C. junos) is a widely cultivated plant in Korea, China, and Japan, known for its fragrant fruit used in Korean cuisine and traditional medicine (Kim et al., 2010). Extracts obtained from various parts of the fruit C. junos, such as the peel, seeds, and pulp, possess diverse chemical compositions and exhibit a range of biological activities (Song et al., 2019).
Extracts derived from C. junos have been found to possess anti-inflammatory, antioxidant, and anti-obesity effects (Shimada, 2015; Zang et al., 2014). Major constituents of C. junos extracts, such as naringin and hesperidin, have demonstrated anti-inflammatory properties in animal models (Nie et al., 2012). The extract from the peel scavenges free radicals and reduces nitric oxide production, while C. junos seed oil, containing unsaturated fatty acids, exhibits anti-inflammatory and antioxidant properties (Hong et al., 2017; Ko et al., 2020; Shin et al., 2010).
Moreover, limonoids derived from C. junos seeds have been shown to have a positive impact on beneficial intestinal microbiota (Minamisawa et al., 2021).
Additionally, Citrus junos essential oil (CJEO) with its unique aroma finds applications in the food, aromatherapy, and cosmetics industries. Inhalation of CJEO has demonstrated effects such as reduced heart rate, increased cerebral blood flow, and sedative and anxiolytic-like effects. CJEO also exhibits antimicrobial properties (El-Toumy & Hussein, 2020; Matsumoto et al., 2014; Satou et al., 2012).
The objective of this study is to evaluate the effects of CJEO on skin anti-aging and establish baseline data for future investigations. The chemical composition of CJEO was determined using gas chromatography-mass spectrometry (GC-MS). Its impact on cell viability, inflammation, collagen degradation, and collagen synthesis were assessed in HEKa and HDF cell lines. Quantitative RT-PCR was employed to measure mRNA levels and identify biomarkers associated with inflammation and skin elasticity in the aging process. Additionally, a quantitative analysis using HPLC was conducted to optimize the formulation with high concentrations of active CJEO ingredients. In vitro efficacy tests were followed by clinical studies assessing CJEO's effectiveness in improving elasticity. Safety evaluations were performed during and after CJEO emulsion application.
Materials and Methods1. Preparation of CJEOIn 2020, C. junos fruits were sourced from Hansung Food Co., Ltd. (Korea). The fruits underwent authentication and were confirmed to be C. junos species. The essential oil was extracted from the fruit peels during the process of pressing the juice from the fruits. Subsequently, the obtained CJEO was stored under refrigeration at 4℃ to ensure sample preservation until the analyses were conducted. For the in vitro efficacy tests, CJEO was dissolved in a 10% dimethyl sulfoxide (DMSO) solution, followed by sonication for 5 minutes to facilitate proper mixing.
2. Cell lines and cultureHuman dermal fibroblasts (HDF) were cultured in Fibroblast Basal Medium (FBM 106) supplemented with low serum growth supplement (LSGS), penicillin (100 IU/mL) and streptomycin (100 μg/mL). Human epidermal keratinocytes (HEKs) were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with penicillin (100 IU/mL) and streptomycin (100 μg/mL), and 10% fetal bovine serum (FBS). Both HDF and HEKs cell lines were maintained in a 5% CO2 incubator at 37℃. Upon reaching confluency, the HDF and HEKa monolayer cultures were detached using 0.25% trypsin-EDTA solution.
3. Chemical composition analysisThe chemical composition of CJEO was analyzed by GC-MS using a Shimadzu TQ-8050NX instrument equipped with a SH-Rxi-5Sil MS column (30 m×0.25 mm, 0.25 μm film). The GC-MS analysis involved the following protocol: and initial column and oven temperature of 50℃, which was gradually increased to 250℃ and held for 3 to 5 minutes. The ion source temperature was set at 250℃, and real-time analytics software was employed. Prior to analysis, the CJEO sample was diluted in CHCl3 and filtered through a 0.45 μm PVDF membrane. A splitless injection of the diluted sample (1.0 μL) was used for analysis. Helium was used as the carrier gas at a flow rate of 1.00 mL/min. Component identification was based on retention indices relative to a homologous series of n-alkanes and mass spectral fragmentation patterns, which were compared with existing literature and an internal MS library. The relative amounts of components were calculated based on GC peak areas using response factors. Detailed analytical conditions for GC/MS can be found detailed in Table 1.
4. Cell viability assayThe viability of HEKs and HDF cells was assessed using a modified Mosmann's method based on the conversion of 3-(4,5-demethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) into formazan through mitochondrial oxidation (Mosmann, 1983). First, 1×105 cells/well were seeded in a 96- well plate and cultured for 18 hours. Then, CJEO dissolved in 10% DMSO and deionized water was added to the cells at concentrations of 0, 10, 50, 100, 500, and 1000 μg/mL. After 24 hours of incubation, the medium was removed, the cells were washed with PBS, and a new medium was added. The cells were then treated with 5 mg/mL MTT solution (Sigma Aldrich Corp., USA). Formazan crystals produced from MTT were solubilized in 150 μL DMSO and the absorbance of each well was measured at 570 nm using a microplate reader (Epoch2C, USA). The optical density of formazan in control cells without CJEO was taken as 100% viability.
5. NO concentrationThe quantification of nitric oxide (NO) production was carried out using the Griess reagent assay (Invitrogen, USA). HEKs were cultured in a 96-well plate for 24 hours, followed by exposure to 200 mJ/cm2 of UVB radiation for 10 minutes. Subsequently, CJEO was added to the wells at concentrations of 0, 5, 10, 50, and 100 μg/mL. After a 48-hour incubation, the supernatants were collected for analysis of NO content. To determine the NO concentration, a 150 μL aliquot of Griess reagent, consisting of 1% sulfanilic acid and 0.25% naphthylethylenediamine dihydrochloride) was added to 20 μL of each sample following the manufacturer's instructions. The absorbance of the resulting mixture was measured at 548 nm using a microplate reader. The NO concentration was calculated by referencing the nitrite standard curve and determining the nitrite concentration corresponding to the mean absorbance value.
6. RNA isolation and quantitative RT-PCR1) The expression analysis of IL-1β, IL-6, NF-κB, and TNF-αTo analyze the expression of IL-1β, IL-6, NF-κB, and TNF-α, the following steps were followed. HEKs cells were incubated in DMEM supplemented with 10% FBS, 100 IU/mL penicillin, 100 μg/mL streptomycin, and maintained in a 5% CO2 environment at 37℃ for 24 hours. Subsequently, the cells were treated with various concentrations of CJEO and cultured for an additional 48 hours. Total RNA was isolated from the cells, and cDNA synthesis was performed using a PrimeScript 1st cDNA Synthesis kit. Amplification was carried out through 35 cycles using a Thermalcycler. The PCR products were visualized on a 1% agarose gel, and the relative gene expression was analyzed using a gel documentation system.
2) The expression analysis of MMP-1, MMP-3, COL1A1, COL3A1To determine the mRNA expression of MMP-1, MMP-3, COL1A1, and COL3A1, HDF cells were cultured in Medium 106 supplemented with 1×LSGS, 100 IU/mL penicillin and 100 μg/mL streptomycin in a 5% CO2 environment at 37℃ for 24 hours. After removing the medium the fibroblasts were treated with various concentrations of CJEO (5, 10, 50, 100 μg/mL) and cultured for 48 hours. Total RNAs from each well were isolated using the TransZol reagent. The obtained total RNA was used to generate the cDNA using the PrimeScript 1st cDNA Synthesis kit, following the manufacturer's instructions. PCR amplifications were performed in a thermalcycler with 35 cycles: denaturation at 95℃ for 1 minute, annealing at 50 to 60℃ for 30 seconds, and extension at 72℃ for 1 minute. The primer sequences and further details of PCR are provided in Table 2. GAPDH served as a control gene. PCR products were electrophoresed on a 1% agarose gel and visualized using ethidium bromide staining. A gel documentation system was utilized to analyze the results and determine the relative ratio of changes in the target genes compared to the control.
7. Clinical studiesThe study protocol and the informed consent form were approved by an independent regional ethical committee (DrSSL-2021-56; Dermacosmetics Institutional Biosafety and Ethics Committee, Korea), and written informed consent was obtained from all participants before their participation in this research. This study included only female participants, with a total of 20 to 60 years of age (mean age 39.1 years), who were recruited to ensure a comprehensive evaluation. An oil-in-water (O/W) emulsion was meticulously developed, incorporating specially selected ingredients to enhance and maintain skin homeostasis. The emulsion formulation comprised CJEO, identified as the O/W emulsion type with the highest CJEO concentration determined in the previous HPLC quantification study. In addition, a placebo emulsion without CJEO was also formulated.
For dermal patch testing, three emulsions with different concentrations of CJEO were prepared. In the primary skin irritation patch test, each formulation was individually applied to participants' skin. The test products included the placebo emulsion (without CJEO), 0.15% CJEO emulsion, and 0.3% CJEO emulsion. All formulations were prepared under similar conditions as outlined in Table 3.
Based on the results of the dermal patch tests, the placebo emulsion and 0.3% CJEO emulsion, both demonstrating no skin irritation, were selected for the subsequent 4-week clinical trial.
The clinical trial consisted of three visits: an initial screening, scoring, and evaluation visit, followed by evaluations at week 2 and week 4. After obtaining informed consent and medical history, participants underwent screening for inclusion and exclusion criteria. Random assignment was conducted, with participants being assigned to either the control group (placebo emulsion) or the experimental group (CJEO emulsion). Blinding of the test materials was ensured. Participants were instructed to apply the test products to their inner arms, using gentle circular motions, every morning and night for 4 weeks after thoroughly washing and drying their hands. The test area remained free of other topical treatments.
During each visit, tolerance and elasticity evaluations were conducted in the same designated areas. Skin elasticity was objectively measured using a Cutometer dual MPA 580. The efficacy of the test products was assessed based on elasticity measurements (E, mm) after 2 weeks and 4 weeks. The condition of the skin and evaluation parameters were recorded, and any adverse events were carefully documented throughout the study.
8. Statistical analysisFor the in vitro experiments, mean±standard deviation (SD) was presented based on at least three independent experiments. Data analysis employed the student's t-test using Microsoft Excel 365 (Microsoft, USA). A significance level of p<0.05 was deemed indicative of statistical significance.
In the context of the clinical trial, a cohort of at least 20 participants was recruited. Rigorous statistical analysis was applied to assess the impact of the tested products on pre- and post-product usage data, with significance set at p<0.05. To validate the statistical analyses, tests were performed to confirm the assumptions of normality and sphericity. This encompassed the use of both parametric methods, such as Two-way Repeated Measures ANOVA and Two-way Repeated Measure MANOVA, as well as a non-parametric approach utilizing the LD.F2 test.
Additionally, post-hoc analyses were conducted, employing the Bonferroni correction method to adjust for multiple comparisons. These meticulous statistical techniques were thoughtfully chosen to ensure the robustness and reliability of our findings, adhering to the highest scientific standards.
Results1. Chemical Composition of CJEOThe chemical composition of CJEO was analyzed using gas chromatography-mass spectrometry (GC-MS), which identified a total of 26 volatile components. These components were classified into 7 oxygenated terpenes and 19 non-oxygenated terpenes, with 12 being monoterpenes and 7 being sesquiterpenes. Among the identified constituents, the major compounds in CJEO were α-pinene, β-myrcene, p-cymene, d-limonene, γ-terpinene, linalool, and β-farnesene. The relative proportions of each component were determined based on GC peak area. The quantitative data for the major components is presented in Table 4.
2. Effect of CJEO on cell viabilityTo assess the impact of CJEO on cell viability, the MTT assay was performed on HEKa and HDF cell lines. The results showed a dose-dependent decrease in cell viability with increasing concentrations of CJEO across all three cell lines. Particularly, the most significant reduction in cell viability was observed at a concentration of 500 μg/mL of CJEO. For subsequent experiments, a concentration of 100 μg/mL of CJEO, which exhibited approximately 90% cell viability, was selected.
3. Inhibition of nitric oxide (NO) productionThe inhibitory effect of CJEO on nitric oxide (NO) production was evaluated in HEKa cells. Treatment with CJEO lead to a notable reduction in NO production compared to the untreated control group. While all tested concentrations of CJEO displayed a decrease in NO production, the inhibitory effect was relatively weaker than that of the positive control, allantoin (Figure 1). These findings suggest that CJEO possesses the potential to suppress NO production in HEKa cells, indicating its anti-inflammatory properties.
4. Effects of CJEO on gene expression1) Expressions of TNF-α, IL-1β, IL-6, and NF-κBTo evaluate the impact of CJEO on mRNA expression in UV-induced HEKs cells, the expression levels of TNF-α, IL-1β, IL-6, and NF-κB were measured using quantitative RT-PCR (qRT-PCR). The results showed that CJEO significantly inhibited the expressions of TNF-α, IL-1β, IL-6, and NF-κB in a dosedependent manner (p<0.05). However, it is important to note that the anti-inflammatory activity of CJEO was relatively weaker when compared to the 50 μg/mL concentration of allantoin, as depicted in Figure 2. Nevertheless, these findings demonstrate that CJEO treatment effectively reduces the expression of inflammatory biomarkers in a dose-dependent manner.
2) Expressions of MMP1, MMP3, COL1A1, and COL3A1To determine the impact of CJEO on mRNA expression in HDF cells, the expressions of collagen degradation-related genes, MMP1 and MMP3, were assessed using quantitative RT-PCR (qRT-PCR).
The results demonstrate that CJEO dose-dependently inhibits the expression of MMP1 and MMP3, as illustrated in Figure 3. At a concentration of 100 μM/mL, CJEO exhibited greater inhibitory effects on MMP1 and MMP3 expression compared to the 50 μM concentration of retinyl palmitate.
Furthermore, the expressions of collagen synthesis-related genes, COL1A1 and COL3A1, were also assessed using quantitative RT-PCR (qRT-PCR) in HDFs.
Treatment with CJEO at concentrations of 50, and 100 μg/mL resulted in a significant increase in COL1A1 expression to 125.56%, and 163.67%, respectively (p<0.05). The positive control treatment with retinyl palmitate (50 μM) significantly increased COL1A1 expression to 186.37%, as depicted in Figure 3.
Similarly, treatment with CJEO at concentrations of 10, 50, and 100 μg/mL resulted in a significant increase in COL3A1 expression to 101.47%, 125.22%, and 137.89%, respectively (p<0.05). The positive control also significantly increased COL3A1 expression to 130.44%, as shown in Figure 3.
Overall, the expressions of COL1A1 and COL3A1 were significantly increased by CJEO in a dose-dependent manner (p<0.05).
5. Dermal patch testA dermal patch test was conducted using an IQ Ultimate patch loaded with 20 μL of CJEO emulsion at two different concentrations (0.15 and 0.3%). The patches were applied to the backs of 30 participants for 24 hours. Skin reactions were assessed by a single trained assessor at 1 and 24 hours after patch removal, using modified York's methods (York et al., 1995). The assessment included evaluation erythema, spots, edema, and vesicles. Each skin reaction was given a score ranging from 0 to 4, where a score of "0" indicated no visible reaction, and higher scores represented increasing severity of erythema, edema, and vesicular erosion. The scoring was done on a simple scale, e.g. 0, +, ++, +++, ++++ as described in Table 5.
The dermal irritation index was calculated using the following equation.
The mean dermal irritation index was categorized into the following levels: No irritation for scores between 0.00 and 0.25, slight irritation for scores between 0.26 and 1.00, moderate irritation for scores between 1.01 and 2.50, and severe irritation for scores between 2.51 and 4.00. In this study, all participants who underwent the dermal irritation index assessment at the concentrations of 0.15% and 0.3% of CJEO obtained a dermal irritation index of 0.00. These consistent results from the two assessments for primary skin irritation, across all participants, provide compelling evidence suggesting a lack of significant irritation potential associated with the CJEO emulsion at these concentrations.
6. Skin elasticity assessmentAfter randomly allocation of participants' left and right anterior forearms into the control and experimental groups, both groups used their respective products for 4 weeks, after which skin elasticity (E, mm) was measured. As shown in Table 6, the experimental group exhibited a significantly greater improvement in skin elasticity (E, mm) compared to the control group. These findings are consistent with the observations made after randomly allocating participants' left and right anterior forearms into the control and experimental groups, with both groups using their respective products for 4 weeks before measuring skin elasticity (E, mm). The statistical significance of this difference was confirmed (p<0.05), providing comprehensive insights into the efficacy of the experimental product.
7. Safety evaluationDuring the study, participants did not report any discomfort during the application or post-application period. Additionally, no persistent erythema or post-inflammatory hyperpigmentation was observed. The CJEO emulsion was well-tolerated, and no adverse effects were reported throughout the study.
ConclusionsThe exploration into the biological efficacy of CJEO as a bioactive ingredient for anti-aging products divulged its potent anti-aging capabilities through an integrative approach encompassing chemical composition analysis, in vitro experimentation, and clinical trials. The chemical composition analysis through GC-MS revealed 26 volatile components within CJEO, categorized into 7 oxygenated terpenes and 19 non-oxygenated terpenes, including 12 monoterpenes and 7 sesquiterpenes. Noteworthy compounds encompassed α-pinene, β-myrcene, p-cymene, d-limonene, γ-terpinene, linalool, and β-farnesene.
CJEO exhibited notable inhibition of NO production, and qRT-PCR analysis illuminated its potential in mitigating skin inflammation through decreased pro-inflammatory cytokines and augmented collagen synthesis markers, indicative of anti-inflammatory and skin-firming properties. However, to deepen comprehension, further investigations should delve into each specific constituent's effects and potential synergistic interactions.
Furthermore, clinical trials underscored CJEO emulsion's efficacy in enhancing skin elasticity. While both CJEO and placebo emulsions boosted skin elasticity, the former showcased significant advancement from weeks 2 to 4 post-application, in contrast to the placebo group. Despite debates around limonene, a major CJEO component, pertaining to possible skin irritation, our clinical trial established the CJEO emulsion's safety through skin irritation tests and comprehensive assessments. In essence, this study confirms CJEO's prowess as an anti-aging ingredient.
This work is part of Byel Kim's Ph.D. thesis at Konkuk University, Seoul, Korea.
NOTESFigure 1.Effect of Citrus junos essential oil on nitric oxide (NO) production in HEKa.NO production levels were assessed following treatment with increasing concentrations of CJEO. Allantoin (50 μg/mL) was used as a positive control to compare the impact of CJEO on NO production. Each value presents the mean±SD of three independent experiments. Statical significance was determined compared to the UVB-treated group (*p<0.05).
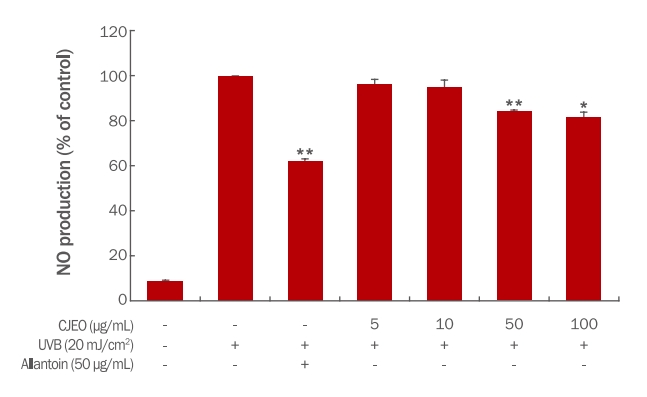
Figure 2.Effect of Citrus junos essential oil on NF-κB, IL-1β, IL-6, and TNF-α in HEKs cellsThe expression levels of NF-κB (A), IL-1β (B), IL-6 (C), and TNF-α (D) in HEKs cells treated with increasing concentrations of CJEO were compared to the untreated control using qRT-PCR. Allantoin (50 μg/mL) was used as a positive control. Each value presents the mean ±SD of three independent experiments. Statistical significance was determined by comparing each treatment group to the untreated control (*p<0.05).
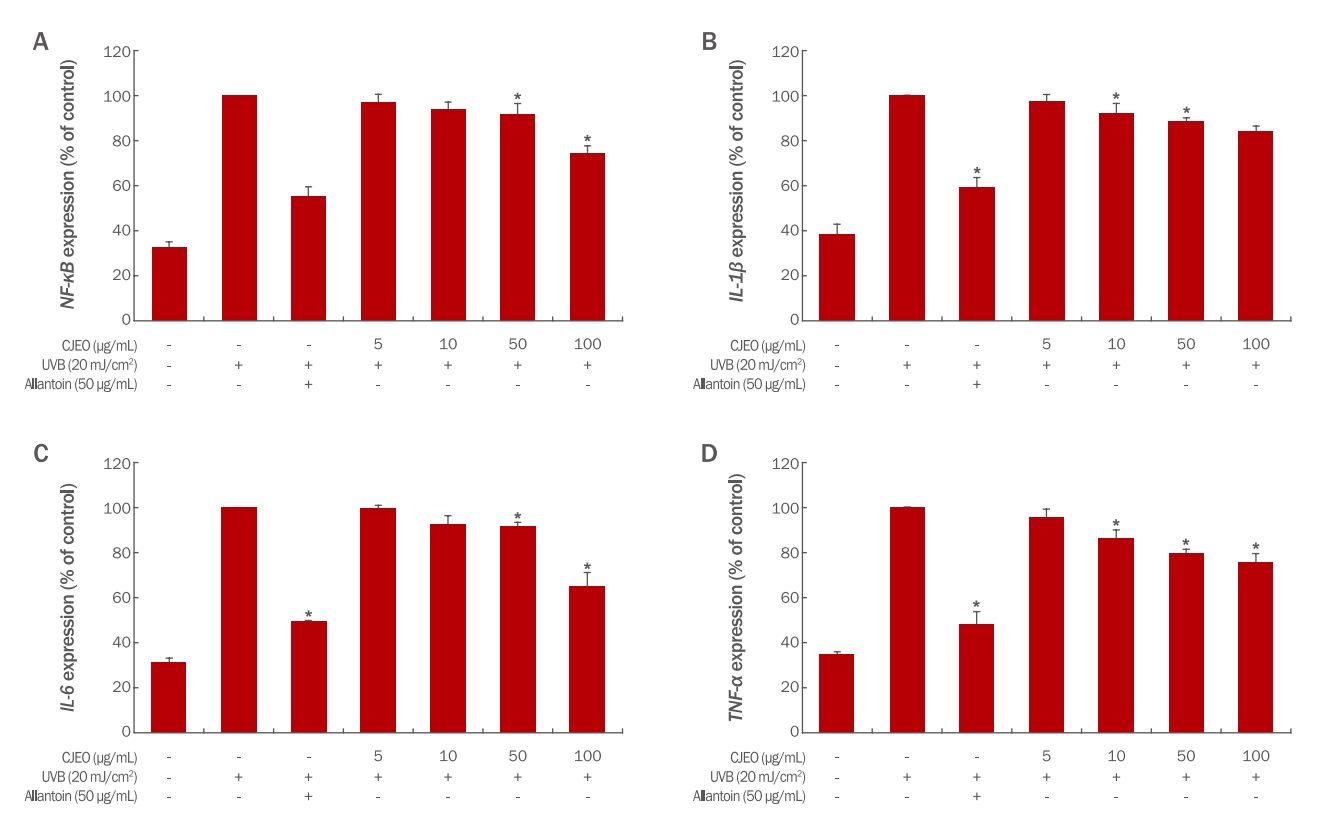
Figure 3.Effect of Citrus junos essential oil on COL1A1, COL3A1, MMP-1, and MMP-3 expression in HDF cells.The expression levels of COL1A1 (A), COL3A1 (B), MMP-1 (C), and MMP-3 (D) were measured by qRT-PCR in HDFs treated with increasing concentrations of CJEO compared to the untreated control. Retinyl palmitate (50 μM) was used as a positive control. Data are presented as the mean±SD from three individual experiments. Statistical significance was determined by comparing each treatment group to the untreated control (*p<0.05).
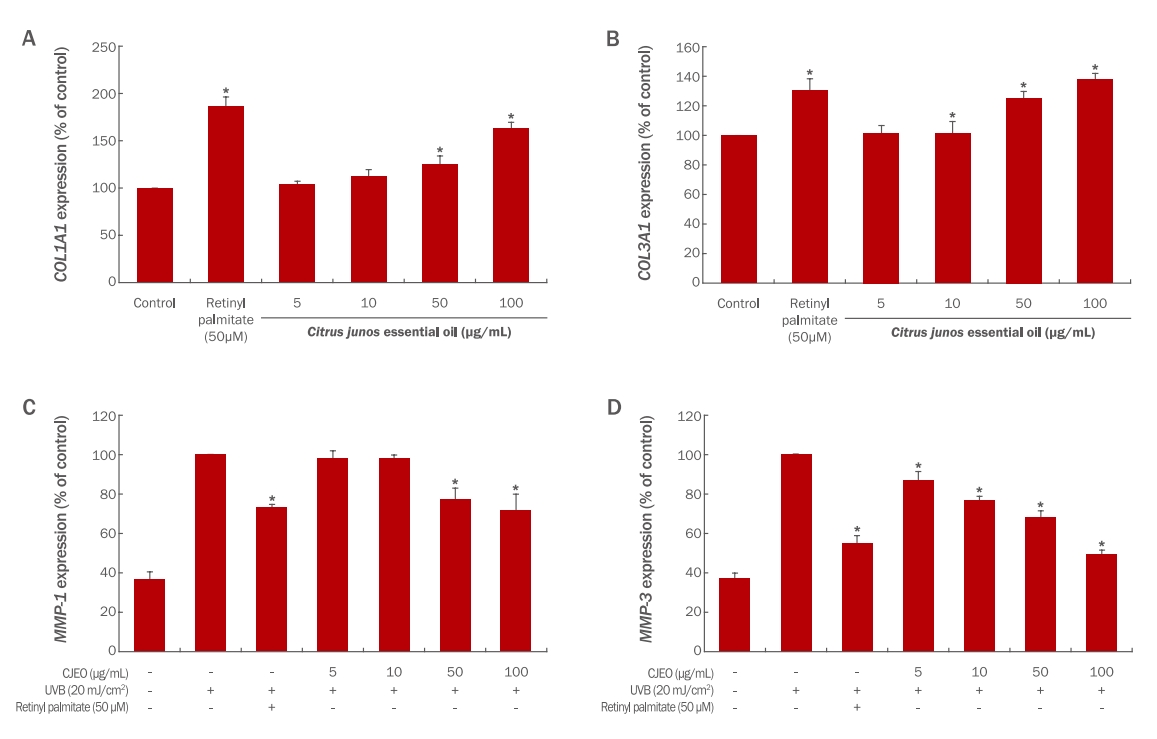
Table 1.Analytical conditions of GC-MS Table 2.Primer sequences (5’→3’)
*2 for HDF; IL-1β , interleukin 1 beta; IL-6 , interleukin 6; TNF-α , tumor necrosis factor-α; NF-κB , Nuclear factor kappa B; COL1A1 , collagen type I alpha 1 chain; COL3A1 , collagen type III alpha 1 chain; MMP-1 , collagenase-1; MMP-3 , stromelysin-1; GAPDH , glyceraldehyde-3-phosphate dehydrogenase. Table 3.Formulation of Placebo and Test emulsion for evaluating the skin anti-aging efficacy in a 4-week clinical trial. Table 4.Chemical composition of Citrus junos essential oil (CJEO) Table 5.Grading description of the irritant response Table 6.Comparison of skin elasticity (E, mm) changes in experimental and control groups ReferencesAbbas H, Kamel R, El-Sayed N. Dermal anti-oxidant, anti-inflammatory and anti-aging effects of compritol ATObased resveratrol colloidal carriers prepared using mixed surfactants. International Journal of Pharmaceutics 541: 37-47. 2018.
Ali B, Al-Wabel NA, Shams S, Ahamad A, Khan SA, Anwar F. Essential oils used in aromatherapy: a systemic review. Asian Pacific Journal of Tropical Biomedicine 5: 509-598. 2015.
Ansary TM, Hossain MR, Kamiya K, Komine M, Ohtsuki M. Inflammatory molecules associated with ultraviolet radiation-mediated skin aging. International Journal of Molecular Sciences 22: 3974. 2021.
Arena ME, Alberto MR, Cartagena E. Potential use of Citrus essential oils against acute respiratory syndrome caused by coronavirus. Journal of Essential Oil Research 33: 1-12. 2021.
Bashir MM, Sharma MR, Werth VP. UVB and proinflammatory cytokines synergistically activate TNF-α production in keratinocytes through enhanced gene transcription. Journal of Investigative Dermatology 129: 994-1001. 2009.
Borg M, Brincat S, Camilleri G, Schembri-Wismayer P, Brincat M, Calleja-Agius J. The role of cytokines in skin aging. Climacteric 16: 514-521. 2013.
Buchbauer G, Jirovetz L. Aromatherapy—use of fragrances and essential oils as medicaments. Flavour and Fragrance Journal 9: 217-222. 1994.
Burnett CL, Fiume MM, Bergfeld WF, Belsito DV, Hill RA, Klaassen CD, Liebler DC, Marks JG, Shank RC, Slaga TJ, et al. Safety assessment of Citrus-derived peel oils as used in cosmetics. International Journal of Toxicology 38: 33-59. 2019.
Dunn JH, Koo J. Psychological stress and skin aging: a review of possible mechanisms and potential therapies. Dermatology Online Journal 19: 1-18. 2013.
El-Toumy SAH, Hussein AA. Cold pressed yuzu (Citrus junos Sieb. ex Tanaka) oil. Cold pressed oils. Elsevier Inc. pp711-718. 2020.
Firenzuoli F, Jaitak V, Horvath G, Bassolé IHN, Setzer WN, Gori L. Essential oils: new perspectives in human health and wellness. Evidence-Based Complementary and Alternative Medicine 2014: 467363. 2014.
Fukumoto S, Morishita A, Furutachi K, Terashima T, Nakayama T, Yokogoshi H. Effect of flavour components in lemon essential oil on physical or psychological stress. Stress and Health 24: 3-12. 2008.
Ganceviciene R, Liakou AI, Theodoridis A, Makrantonaki E, Zouboulis CC. Skin anti-aging strategies. Dermatoendocrinology 4: 308-319. 2012.
Hong YS, Lee YS, Kim KS. Comparison of volatile flavor compounds of yuzu, kumquat, lemon and lime. Korean Journal of Food Preservation 24: 394-405. 2017.
Hongratanaworakit T. Physiological effects in aromatherapy. Songklanakarin. Journal of Science and Technology 26: 117-125. 2004.
Je YR, An SK, Ro HS, Cho JW, Bae SH. Effects of the essential oils of sweet orange, lavender and amyris on EEG activity. Asian Journal of Beauty and Cosmetology 19: 651-664. 2021.
Jung KH, Lee KJ. A comparative analysis of the volatile components of Agarwood from Vietnam and other regions. Asian Journal of Beauty and Cosmetology 20: 481-498. 2022.
Kang JW, Nam GR, Yoon YH, Kim GM, Bae SW, Lee HJ, Bae SH, Cha YK, Cho HD, Cho HE. Suppression of matrix metallopeptidase-3 expression in human dermal fibroblasts by Decursin from Angelica gigas Nakai root extracts fermented with Jeju lava seawater. Asian Journal of Beauty and Cosmetology 19: 65-76. 2021.
Kazemi M, Mohammadifar M, Aghadavoud E, Vakili Z, Aarabi MH, Talaei SA. Deep skin wound healing potential of lavender essential oil and licorice extract in a nanoemulsion form: Biochemical, histopathological and gene expression evidences. Journal of Tissue Viability 29: 116-124. 2020.
Kim DS, Kim DH, Oh MJ, Lee KG, Kook MC, Park CS. Antiaging and whitening activities of ethanol extract of yuza (Citrus junos SIEB ex TANAKA) by-product. Journal of the Society of Cosmetic Scientists of Korea 36: 137-143. 2010.
Ko EA, Nam SH, Jeong H, Kim BY, Kwak SH, Kim S, Hong IK, Kang H. Antioxidant, anti-inflammatory and anti-allergenic effects of Citrus Junos seed oil and its human skin protection. Journal of the Society of Cosmetic Scientists of Korea 46: 283-294. 2020.
Lago JC, Puzzi MB. The effect of aging in primary human dermal fibroblasts. PLoS One 14: 1-14. 2019.
Martin KI, Glaser DA. Cosmeceuticals: the new medicine of beauty. Missouri Medicine 108: 60-63. 2011.
Matsumoto T, Asakura H, Hayashi T. Effects of olfactory stimulation from the fragrance of the Japanese Citrus fruit yuzu (Citrus junos Sieb. ex Tanaka) on mood states and salivary chromogranin A as an endocrinologic stress marker. Journal of Alternative and Complementary Medicine 20: 500-506. 2014.
Miguel MG. Antioxidant and anti-inflammatory activities of essential oils: A short review. Molecules 15: 9252-9287. 2010.
Minamisawa M, Suzumura T, Bose S, Taniai T, Kawai G, Suzuki K, Yamaguchi A, Yamanaka S. Effect of Yuzu (Citrus junos) seed limonoids and spermine on intestinal microbiota and hypothalamic tissue in the Sandhoff disease mouse model. Medical Sciences 9: 17. 2021.
Mirastschijski U, Lupše B, Maedler K, Sarma B, Radtke A, Belge G, Dorsch M, Wedekind D, McCawley LJ, Boehm G. Matrix metalloproteinase-3 is key effector of TNF-α-induced collagen degradation in skin. International Journal of Molecular Sciences 20: 1-14. 2019.
Modarresi M, Farahpour MR, Baradaran B. Topical application of Mentha piperita essential oil accelerates wound healing in infected mice model. Inflammopharmacology 27: 531-537. 2019.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of Immunological Methods 65: 55-63. 1983.
Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix metalloproteinases: biologic activity and clinical implications. Journal of Clinical Oncology 18: 1135-1149. 2000.
Neves J, Sousa-Victor P. Regulation of inflammation as an anti-aging intervention. The FEBS Journa 287: 43-52. 2020.
Nguyen LP, Sawamura M. Characteristic aroma composition profile of mature stage Citrus junos (Yuzu) peel oil from different origins. Food Science and Technology Research 14: 359-366. 2008.
Nie YC, Wu H, Li PB, Luo YL, Long K, Xie LM, Shen JG, Su WW. Anti-inflammatory effects of naringin in chronic pulmonary neutrophilic inflammation in cigarette smoke-exposed rats. Journal of Medicinal Food 15: 894-900. 2012.
Papakonstantinou E, Roth M, Karakiulakis G. Hyaluronic acid: a key molecule in skin aging. Dermato-Endocrinology 4: 253-258. 2012.
Parente ME, Ochoa Andrade A, Ares G, Russo F, Jiménez-Kairuz A. Bioadhesive hydrogels for cosmetic applications. International Journal of Cosmetic Science 37: 511-518. 2015.
Pittayapruek P, Meephansan J, Prapapan O, Komine M, Ohtsuki M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. International Journal of Molecular Sciences 17: 868. 2016.
Quan T, Qin Z, Xia W, Shao Y, Voorhees JJ, Fisher GJ. Matrix-degrading metalloproteinases in photoaging. Journal of Investigative Dermatology Symposium Proceedings 14: 20-24. 2009.
Satou T, Miyahara N, Murakami S, Hayashi S, Koike K. Differences in the effects of essential oil from Citrus junos and (+)-limonene on emotional behavior in mice. Journal of Essential Oil Research 24: 493-500. 2012.
Sharmeen JB, Mahomoodally FM, Zengin G, Maggi F. Essential oils as natural sources of fragrance compounds for cosmetics and cosmeceuticals. Molecules 26: 666. 2021.
Shen X, Guo M, Yu H, Liu D, Lu Z, Lu Y. Propionibacterium acnes related anti-inflammation and skin hydration activities of madecassoside, a pentacyclic triterpene saponin from Centella asiatica. Bioscience, Biotechnology and Biochemistry 83: 561-568. 2019.
Shimada A. Antioxidant activity and lipase and alpha-glucosidase inhibitory activities of Yuzu juice (Citrus junos Tanaka). Journal of Yasuda Women’s University 43: 351-357. 2015.
Shin JH, Yang SM, Kang MJ, Lee SJ, Kim SH, Sung NJ. Biological activities of hot water extracts made from Yuza (Citrus junos SIEB ex TANAKA) peel cultivated in Namhae. Korean Journal of Food and Cookery Science 26: 79-87. 2010.
Song HY, Jo A, Shin J, Lim EH, Lee YE, Jeong DE, Lee M. Anti-inflammatory activities of isogosferol, a furanocoumarin isolated from Citrus junos seed shells through bioactivity-guided fractionation. Molecules 24: 1-16. 2019.
Tam I, Stępień K. Secretion of proinflammatory cytokines by normal human melanocytes in response to lipopolysaccharide. Acta Biochimica Polonica 58: 507-511. 2011.
Varani J, Spearman D, Perone P, Fligiel SEG, Datta SC, Wang ZQ, Shao Y, Kang S, Fisher GJ, Voorhees JJ. Inhibition of type I procollagen synthesis by damaged collagen in photoaged skin and by collagenase-degraded collagen in vitro. American Journal of Pathology 158: 931-942. 2001.
Wang F, Garza LA, Cho S, Kafi R, Hammerberg C, Quan T, Hamilton T, Mayes M, Ratanatharathorn V, Voorhees JJ, Fisher GJ, Kang S. Effect of increased pigmentation on the antifibrotic response of human skin to UV-A1 phototherapy. Archives of Dermatology 144: 851-858. 2008.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||